
Getting a COVID-19 test amid the ongoing pandemic is about to become a lot easier.
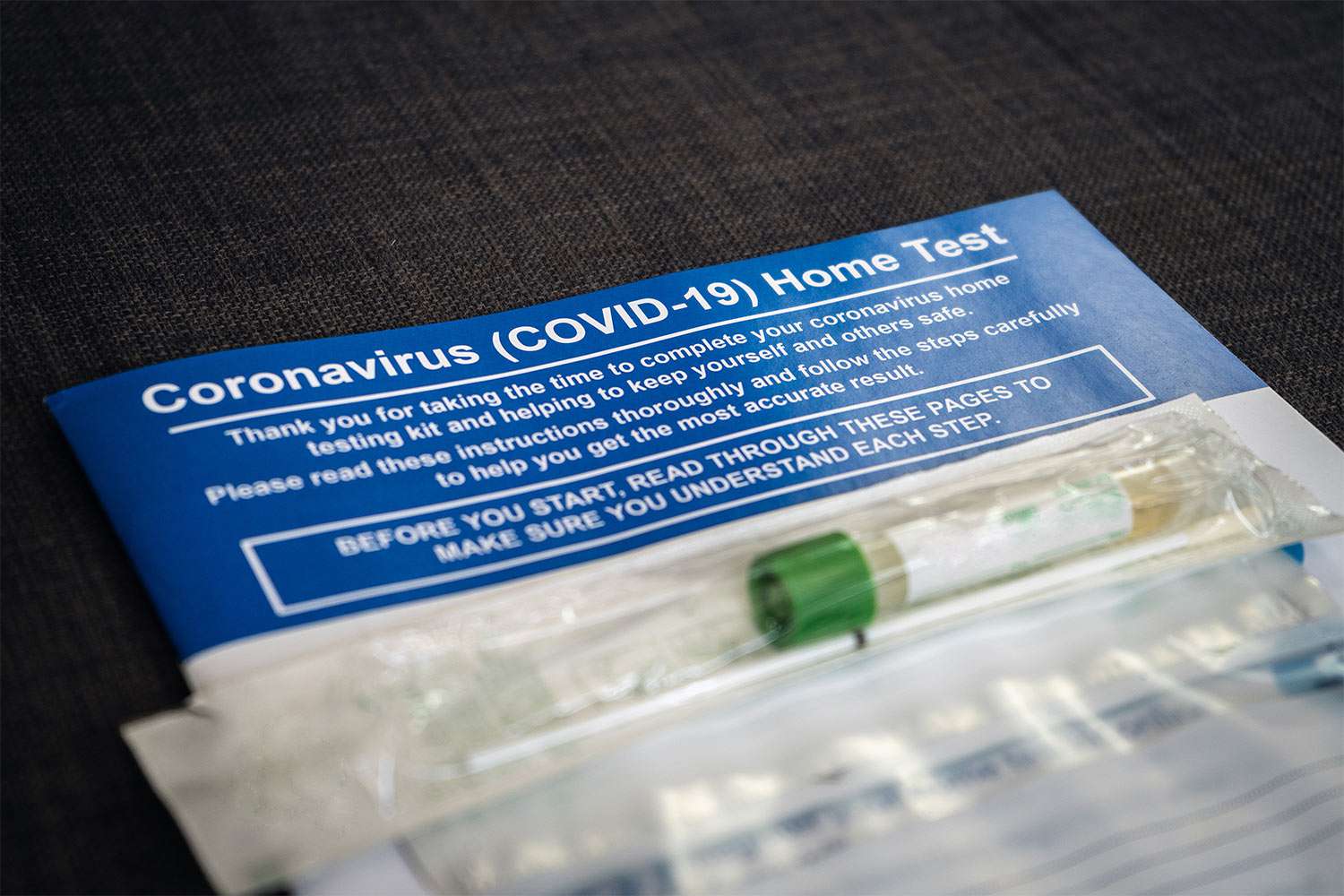
On Tuesday, the Food and Drug Administration (FDA) authorized the first at-home COVID-19 test that doesn’t require a prescription. It will soon become available to purchase nationwide, according to a press release from the organization.
Per the release, the over-the-counter Ellume COVID-19 Home Test detects COVID-19 from a nasal swab sample and takes 15 to 20 minutes to provide a result. The Ellume test can be used by anyone over the age of 2.
Unlike other similar products, the Ellume test can also be utilized by individuals with or without symptoms of COVID-19.
A company spokesperson told the Associated Press that the new at-home test will be available at pharmacies and for purchase online, and will be priced around $30.
"Today’s authorization is a major milestone in diagnostic testing for COVID-19," said FDA Commissioner Stephen M. Hahn, M.D. "By authorizing a test for over-the-counter use, the FDA allows it to be sold in places like drug stores, where a patient can buy it, swab their nose, run the test and find out their results in as little as 20 minutes."
Hahn added: "As we continue to authorize additional tests for home use, we are helping expand Americans' access to testing, reducing the burden on laboratories and test supplies, and giving Americans more testing options from the comfort and safety of their own homes."
Never miss a story — sign up for PEOPLE's free daily newsletter to stay up-to-date on the best of what PEOPLE has to offer, from juicy celebrity news to compelling human interest stories.
"The FDA strongly supports innovation in test development and we have worked tirelessly with test developers to support the shared goal of getting more accurate and reliable tests to Americans who need them," noted Jeff Shuren, M.D., J.D., director of FDA’s Center for Devices and Radiological Health. "Today is a promising step forward and we are eager to continue advancing additional innovation in COVID-19 testing that the science supports."
"This test, like other antigen tests, is less sensitive and less specific than typical molecular tests run in a lab," he continued. "However, the fact that it can be used completely at home and return results quickly means that it can play an important role in response to the pandemic."
Ellume said that it plans to produce millions of tests next month, per the AP.
According to the product's online listing, the at-home testing kit comes with a sterile nasal swab, a dropper and processing fluid, as well as an analyzer that connects to an individual's smartphone through Bluetooth and an associated app.
After administering the test, the application will share the users' results and even offer them an opportunity to connect with a healthcare professional. The app also sends data to health officials, who can track positive cases.
"This is the first test which is really designed to be a true at-home test yourself and obtain a result," Sean Parsons, the company's CEO, told NPR in an interview prior to the product's authorization. "This could be used for people to test themselves, for example, before going to a sporting event or a concert or going to a church to decrease the chance that they spread it other people."
But, per the FDA release, "a small percentage of positive and negative results from this test may be false," as is the case with other COVID-19 tests.
The Ellume approval comes after the FDA approved the first rapid at-home coronavirus test, which can give users results within the hour, last month.
According to the agency, the Lucira COVID-19 All-In-One Test Kit can be obtained with a prescription for patients 14 or older, and will return results in about 30 minutes. The test can also be administered to patients of all ages at a "point-of-care (POC) setting," such as a hospital or clinic.
The test is done by "swirling the self-collected sample swab in a vial that is then placed in the test unit." After 30 minutes or less, the results appear on the test unit's light-up display that indicates whether a person is positive or negative.
On their website, Lucira Health states that the tests have a sensitivity (how well it detects positive cases) of about 94.1 percent and a specificity (how well it detects negative cases) of 98 percent.
As information about the coronavirus pandemic rapidly changes, PEOPLE is committed to providing the most recent data in our coverage. Some of the information in this story may have changed after publication. For the latest on COVID-19, readers are encouraged to use online resources from the WHO and local public health departments. PEOPLE has partnered with GoFundMe to raise money for the COVID-19 Relief Fund, a GoFundMe.org fundraiser to support everything from frontline responders to families in need, as well as organizations helping communities. For more information or to donate, click here.
Source: Read Full Article


