Although autism is a common neurodevelopmental disorder, the multiple factors behind its onset are still not fully understood. Animal models of idiopathic autism, especially mice, are often used to help researchers understand the complicated mechanisms behind the disorder, with BTBR/J being the most commonly used mouse model in the world.
Now, an international research collaboration including Kobe University’s Professor Takumi Toru and Researcher Chia-wen Lin and colleagues have made new discoveries regarding autism onset in mouse models.
In their detailed series of experiments and analyses of BTBR/J mice and the other subspecies BTBR/R, they revealed that endogenous retrovirus activation increases a fetus’s susceptibility to autism. They also discovered that BTBR/R exhibits autistic-like behaviors without reduced learning ability, making it a more accurate model of autism than the widely-used BTBR/J model. These research results were published in Molecular Psychiatry on March 7, 2023.
It is hoped that further research will contribute towards better classification of autism types, as well as the creation of new treatment strategies for neurodevelopmental disorders.
Autism (autism spectrum disorder) is a neurodevelopmental disorder that remains largely unexplored despite the rapidly increasing number of patients. Reasons for this continuing increase in people diagnosed with autism include changes to diagnostic criteria and older fathers becoming more common.
Autism is strongly related to genetic factors and can be caused by abnormalities in DNA structure, such as copy number variations. Animal models, especially mice, are often used in research to illuminate the pathology of autism. Among these models, BTBR/J is a mouse model of the natural onset of autism that is commonly used. Studies have reported various abnormalities in BTBR/J mice including impairment of the corpus callosum (which connects the left and right hemispheres of the brain) and excessive immune system signaling. However, it is not fully understood why this particular lineage displays autistic-like behavioral abnormalities.
The aim of the current study was to shed light on the onset mechanism of these autistic-like behavioral abnormalities by conducting comparative analysis on BTBR/J and its subspecies BTBR/R.
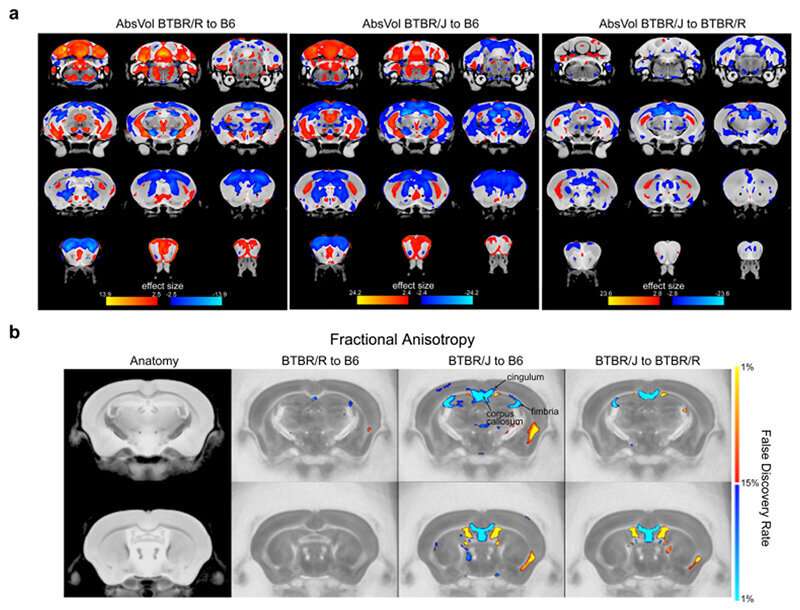
First of all, the researchers conducted MRI scans on BTBR/J and BTBR/R mice to investigate structural differences in each region of the brain. The results revealed that there were differences between BTBR/J and BTBR/R mice in 33 regions including the amygdala. A particularly prominent difference discovered was that even though BTBR/J’s corpus callosum is impaired, BTBR/R’s is normal (Fig. 1).
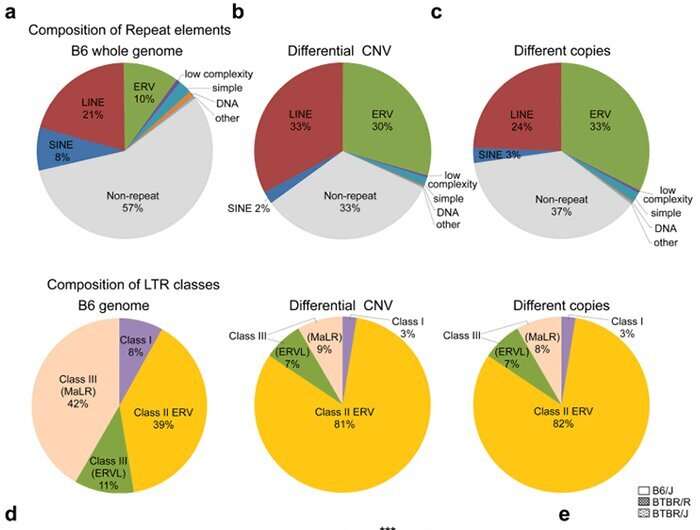
Next, the research group used the array CGH method to compare BTBR/R’s copy number variations with that of a normal mouse model (B6). They revealed that BTBR/R mice had significantly increased levels of endogenous retroviruses (ERV) in comparison to B6 mice (Fig. 2 a–c). Furthermore, qRT-PCR tests revealed that these retroviruses were activated in BTBR/R mice (as shown in Fig. 2d). On the other hand, in B6 mice there was no change in the expression of LINE ERV (which is classified in the same repetitive sequence), indicating that this retroviral activation is specific to BTBR (Fig. 2e).
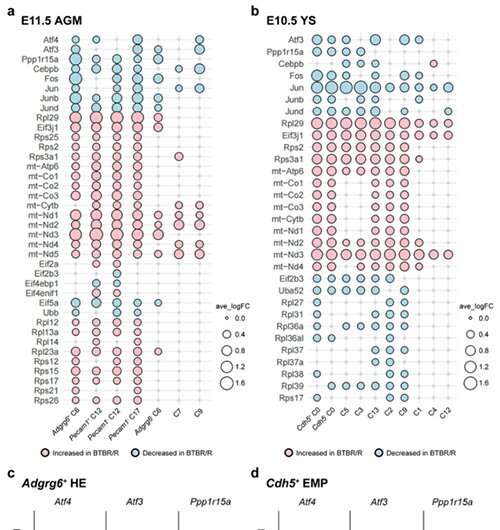
Subsequently, the researchers carried out single-cell RNA analysis on the tissue of embryonic BTBR mice (on the AGM and yolk sac). The results provide evidence of ERV activation in BTBR mice, as expression changes were observed in a group of genes downstream of ERV (Fig. 3).
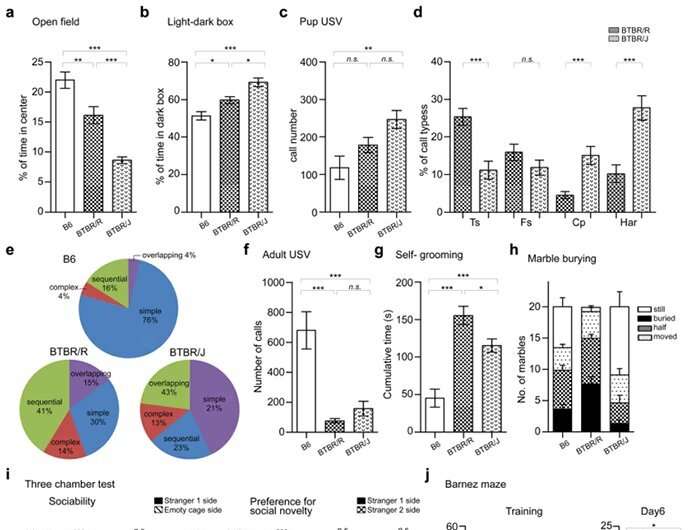
Lastly, the researchers comprehensively investigated the differences between BTBR/J and BTBR/R on a behavioral level. BTBR/R mice were less anxious than BTBR/J and showed qualitative changes in ultrasound vocalizations, which are measured as a way to assess communicative ability in mice (Fig. 4a–f). BTBR/R mice also exhibited more self-grooming behaviors and buried more marbles in the marble burying test (Fig. 4g, h). These two tests were designed to detect repetitive behavioral abnormalities in autistic individuals.
From the results, it was clear that BTBR/R exhibits more repetitive behaviors (i.e., it is more symptomatic) than BTBR/J. The 3-chamber social interaction test, which measures how closely a mouse will approach another mouse, also revealed more pronounced social deficits in BTBR/R than BTBR/J mice (Fig. 4i). In addition, a Barnes maze was used to conduct a spatial learning test, in which BTBR/J mice exhibited reduced learning ability compared to B6 (normal mice). BTBR/R mice, on the other hand, exhibited similar ability to B6 (Fig. 4j).
Overall, the study revealed that retrovirus activation causes the copy number variants in BTBR mice to increase, which leads to the differences in behavior and brain structure seen in BTBR/J and BTBR/R mice (Fig. 5).
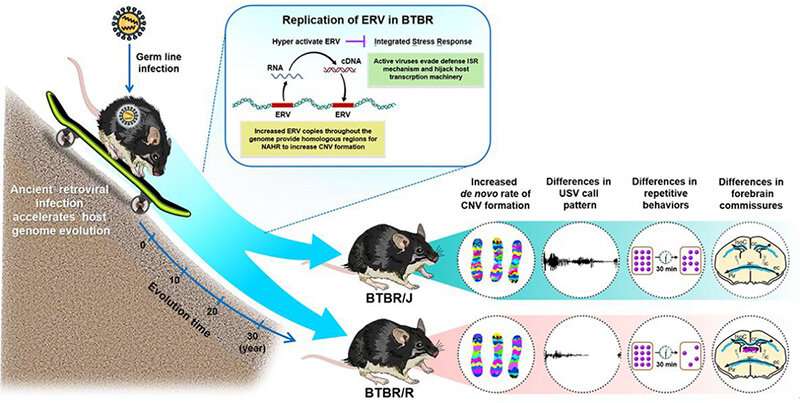
BTBR/J mice are widely used by researchers as a mouse model of autism. However, the results of this study highlight the usefulness of the other lineage of BTBR/R mice because they exhibit autistic-like behavior without compromised spatial learning ability. The results also suggest that it may be possible to develop new treatments for autism that suppress ERV activation. Furthermore, it is necessary to classify autism subtypes according to their onset mechanism, which is a vital first step towards opening up new avenues of treatment for autism.
More information:
Chia-Wen Lin et al, An old model with new insights: endogenous retroviruses drive the evolvement toward ASD susceptibility and hijack transcription machinery during development, Molecular Psychiatry (2023). DOI: 10.1038/s41380-023-01999-z
Journal information:
Molecular Psychiatry
Source: Read Full Article


