Because of the nature of organs like the stomach and liver in the abdominal cavity of mammals, infection can lead to life-threatening sepsis. A visceral adipose tissue called omentum helps cordon off any inflamed or injured areas in the abdominal space. The omentum contains lymphoid aggregates (immune cell clusters) that are referred to as milky spots. Recently, two researchers from Osaka University further examined the molecular and cellular details of how these milky spots develop and mature to better understand their role in the abdominal immune system.
In a newly published article in the Journal of Experimental Medicine, the research team revealed that omental milky spots contain a unique subset of fibroblastic reticular cells (FRCs)—cells that are commonly found in secondary lymphoid organs such as spleen or lymph nodes. Milky spots are known to collect pathogens within the abdomen and then promote an immune response. Yet, their developmental mechanisms are unclear, as previous studies have demonstrated that they do not form in the same manner as secondary lymphoid organs.
“We know that FRCs are critical components of secondary lymphoid organs, displaying a high structural complexity, but the omental milky spots are not as structurally complex,” says Tomomi Yoshihara, lead author of the study. “The diversity of FRCs in milky spots has not yet been well characterized, so we hoped to investigate it in more detail.”
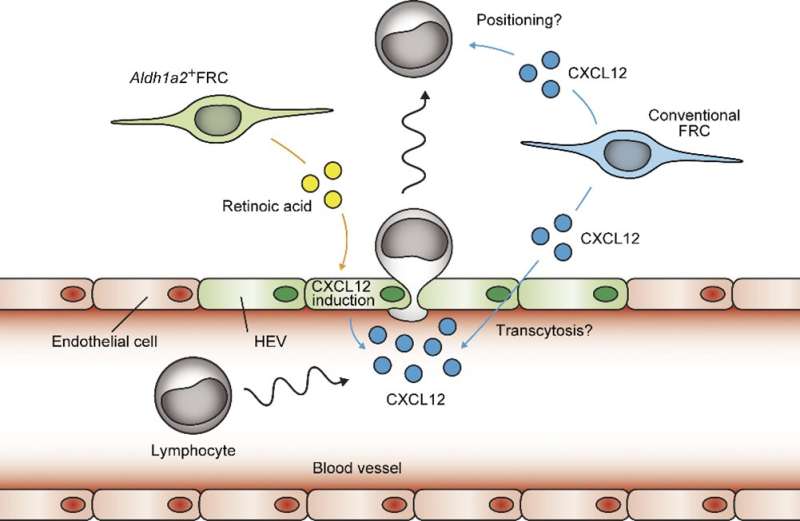
The researchers used a mouse model and various molecular techniques to identify the stromal cells in the omentum. They found a specific subset of FRCs unique to the milky spots, which expresses both Aldh1a2 (the rate-limiting enzyme in retinoic acid synthesis) and Tie2 (an endothelial cell marker). Notably, no cells expressing both Aldh1a2 and Tie2 were present in secondary lymphoid organs, emphasizing their uniqueness to milky spots.
“Retinoic acid is required for many immunological functions,” explains Yasutaka Okabe, senior author of the study. “However, it was unclear what specific role these Aldh1a2-positive FRCs had in the omentum.” When these cells were genetically depleted, the milky spots not only lost their structure and shrunk in size but their ability to recruit specific immune cells was also impaired. “Further mechanistic characterization indicated that Aldh1a2-positive FRCs regulate the display of a molecule called CXCL12 on high endothelial venules (or specialized blood vessels), which recruit certain lymphocytes from blood circulation,” says Tomomi Yoshihara.
Collectively, these data demonstrate that the subset of FRCs unique to omental milky spots are necessary for the entry of lymphocytes to the sites of inflammation or injury in the abdominal cavity. This work will be critical for establishing a deeper understanding of the mechanisms associated with intra-abdominal infections and aid in the development of novel treatment methods for potentially life-threatening scenarios.
More information:
Tomomi Yoshihara et al, Aldh1a2 + fibroblastic reticular cells regulate lymphocyte recruitment in omental milky spots, Journal of Experimental Medicine (2023). DOI: 10.1084/jem.20221813
Journal information:
Journal of Experimental Medicine
Source: Read Full Article


