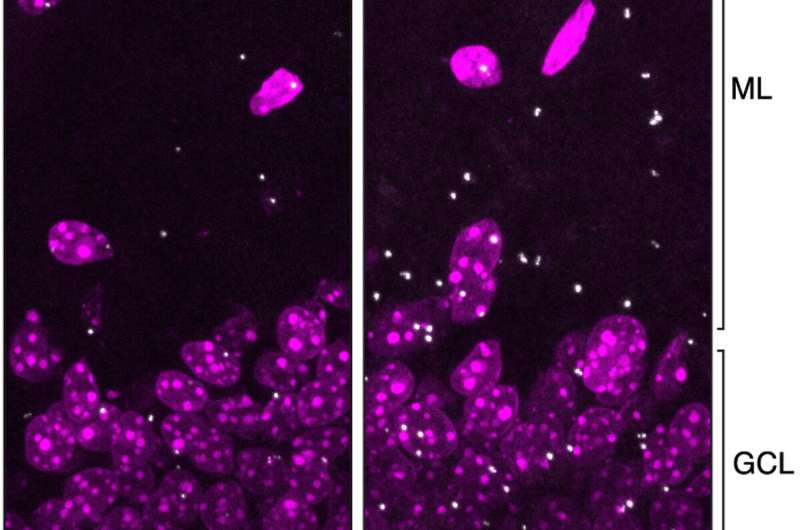
An analysis of human brain cells provides new evidence in support of the “amyloid hypothesis,” the prevailing idea that Alzheimer’s is caused by the accumulation of beta-amyloid proteins in the brain.
In the study, Columbia University researchers found that amyloid sparks an alliance between two proteins in the brain’s neurons and this pairing is linked to about half of the gene changes that are known to occur in the disease, triggering the rapid accumulation of tau proteins, a primary driver of neurodegeneration in the disease.
“This protein pair seems very central to the disease, and because it does not appear to have another function in the brain, it is a good target for a new therapy,” says the study’s senior author, Ulrich Hengst, Ph.D., associate professor of pathology & cell biology (in the Taub Institute for Research on Alzheimer’s Disease and the Aging Brain) at the Columbia University Vagelos College of Physicians and Surgeons.
Protein pair was hidden to previous research
The researchers found the pair when they were looking for proteins that spark hundreds of changes in gene activity that occur in brain cells during Alzheimer’s disease. “Our thought was that if we can interfere with the proteins and prevent those changes, we can prevent the disease,” says Cláudio Gouveia Roque, Ph.D., associate research scientist in the Hengst lab, who conducted the study.
Instead of looking for proteins that act alone, the researchers looked for pairs of different proteins working together.
“We know this type of protein necessarily works in pairs, but previous Alzheimer’s research hadn’t looked for specific pairs. Consequently, our understanding of the changes underlying Alzheimer’s progression has been fragmented and incomplete,” says Hengst. “And because of that, we’ve most likely missed therapeutic opportunities.”
Amyloid causes proteins to stick together
The search by Hengst and Gouveia Roque, together with a previous associate research scientist in the Hengst lab, Jimena Baleriola, uncovered two proteins—ATF4 and CREB3L2—whose binding to each other is triggered by amyloid and that together interact with about 50% of the gene expression changes that occur in brain cells during Alzheimer’s disease.
Once formed, the CREB3L2-ATF4 pair activates a network of other proteins that cause deadly tau deposits to accumulate inside neurons. The protein pair also disables the cellular machinery that clears old and damaging proteins from neurons, another hallmark of Alzheimer’s.
Although CREB3L2 and ATF4 are also found alone in healthy neurons, their binding together is greatly increased in the presence of a stress like excess amyloid, the researchers found.
“These two proteins are like two teenage boys,” says Hengst. “Individually, they may be relatively harmless. But if you put them together without a responsible adult in the room, they’re likely to be up to no good.”
New treatment approach
The findings suggest that Alzheimer’s could be treated by interfering with the CREB3L2-ATF4 pair.
“Normally, proteins that control gene activity are very poor drug targets because they control too many genes. But by targeting this pair we might be able to preserve the function of the two individual proteins while preventing the bad effects of them binding together,” Hengst says.
Hengst and Gouveia Roque have already identified a drug, dovitinib, that interferes with the effects of the protein pair. Dovitinib has been approved by the FDA for the treatment of renal cancer but has not been tested for the treatment of Alzheimer’s. “Nonetheless, the drug is not toxic to neurons and crosses the blood-brain barrier, so this bodes well for future drug development,” Hengst says.
“We’re not talking about getting rid of amyloid with this approach,” adds Gouveia Roque. “If we can interfere with the protein pair, we could slow or perhaps even stop the progression of the disease. Yes, there would still be amyloid in the brain, but the neurons would react far less to it. One could hypothesize that such a drug could be used in combination with an amyloid-reducing drug for even greater effect.”
The paper is published in the journal Science Advances.
More information:
Cláudio Gouveia Roque et al, CREB3L2-ATF4 heterodimerization defines a transcriptional hub of Alzheimer’s disease gene expression linked to neuropathology, Science Advances (2023). DOI: 10.1126/sciadv.add2671
Journal information:
Science Advances
Source: Read Full Article