Goldenhar syndrome is a rare congenital disease, affecting early fetal development. This syndrome includes malformations of varying severity, affecting different parts of the face. Its causes and modes of transmission are still poorly understood.
An international collaboration led by the University of Geneva (UNIGE) and Beihang University, in China, has discovered that pathogenic variants of the FOXI3 gene—responsible for the development of the ear—cause one form of this developmental disorder. The scientists were also able to identify the modes of transmission of the disease when this particular gene is involved. These results have been published in Nature Communications.
First described in 1952 at the University of Geneva (UNIGE) by the ophthalmologist Maurice Goldenhar (1924-2001), Goldenhar syndrome or oculo-auriculo-vertebral dysplasia is a rare congenital disorder with facial asymmetry, malformations of the auditory and ocular systems, and spinal column anomalies, sometimes associated with mental retardation. It varies greatly in expression and severity depending on the individual and is thought to affect approximately one in 3,500 to 5,600 births.
This syndrome is caused by mutations that appear during fetal development. Do these mutations occur spontaneously or is there an inherited form of the disease? The mode of transmission and the molecular mechanisms involved are still poorly understood. A recent international research effort, led by a joint team from the UNIGE and Beihang University, in China, is providing new elements for understanding the disease. The scientists have identified a gene—FOXI3—whose pathogenic variants are involved in one form of the disease.
A gene that builds the ear
“FOXI3 is critically involved in the development of the ear. It is an architect gene: it produces a protein that acts as a transcription factor. In other words, it controls the expression of other genes to start the construction of the ear,” explains Stylianos Antonarakis, Emeritus Professor at the UNIGE Faculty of Medicine and co-last author of the study.
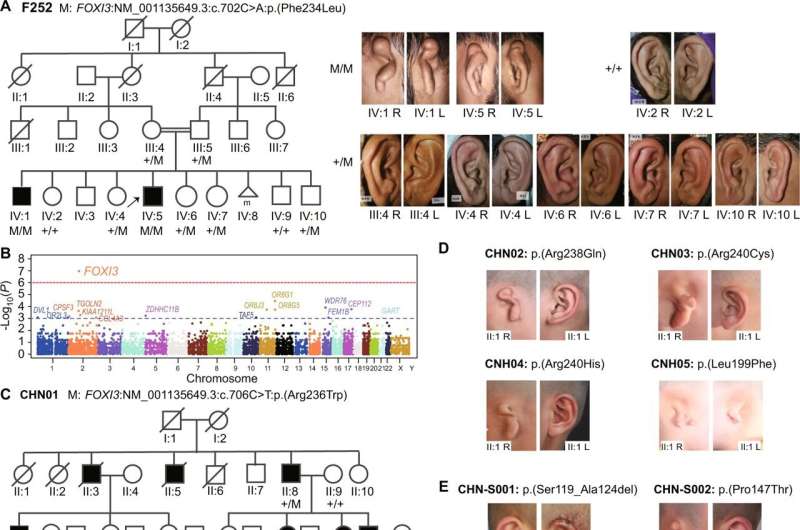
To determine the role of FOXI3, the scientists used the genetic profiles of a highly consanguineous Pakistani family involved in a larger research project on rare diseases. In this family, two brothers have the syndrome but not their parents, nor the other four siblings. Both patients have pathogenic mutations in both copies of the FOXI3 gene, while several other members of this family, who do not have Goldenhar syndrome, have a mutation in only one copy of the gene.
“This first analysis allowed us to confirm that pathogenic mutations in both copies of the FOXI3 gene, each inherited from a parent, are necessary for a form of the disease to develop,” explains Stylianos Antonarakis. This is called autosomal recessive inheritance.
Responsible for one form of the disease
To find out whether pathogenic variants in FOXI3 and their autosomal recessive inheritance are systematically involved, the researchers also analyzed the genetic profiles of 670 unrelated patients in Europe and China.
“Eighteen pathogenic variants in the FOXI3 gene were identified in twenty-one patients. FOXI3 is therefore only one of the genes that can cause the disease, precisely the specific form observed in our Pakistani patients,” explains Ke Mao, researcher at Beihang University and co-first author of the study with Christelle Borel, senior research associate in the Department of Genetic Medicine and Development at the UNIGE Faculty of Medicine, and Muhammad Ansar, senior research and teaching assistant in the same department and head of the Eye Genetics research group at the Jules-Gonin eye hospital.
Another finding that baffled the researchers was that the mode of inheritance, in many other cases, is seemingly autosomal dominant. In this case, only one of the two copies of the gene needs to carry the mutation for the disease to occur. By carefully studying the genome around the FOXI3 gene, scientists have solved this mystery. There is a functional and frequent, but specific, variant of one copy of the FOXI3 gene that, in combination with a severe mutation in the other copy of the FOXI3 gene, also causes the disorder.
“We also created mouse models with mutations in the FOXI3 gene to validate the results obtained in human families. These mice showed facial deformities equivalent to human characteristics,” adds Professor Yong-Biao Zhang of Beihang University, the study’s co-lead author.
These results provide crucial elements for understanding this syndrome and would explain in part the extensive heterogeneity of the disease. They also pave the way for the study of other genes that may be involved in the disease, but also in the healthy development of certain parts of the face, the mechanisms of which are still poorly understood.
More information:
Ke Mao et al, FOXI3 pathogenic variants cause one form of craniofacial microsomia, Nature Communications (2023). DOI: 10.1038/s41467-023-37703-6
Journal information:
Nature Communications
Source: Read Full Article


