Our bodies make blood in a specialized niche—a “nursery” within our bone marrow that nurtures blood stem cells so they can replicate and make different kinds of blood cells. The lab of Leonard Zon, MD, has even shown how blood stem cells, once they settle in the niche, are “cuddled” by nearby cells.
In new work, the Zon Lab, part of the Stem Cell Research program at Boston Children’s, has figured out what ingredients are needed for a good blood stem cell niche and how to create the same conditions outside the bone marrow. Potentially, this could enable blood to be made in other parts of the body when needed to treat blood disorders. The findings were published April 28 in Developmental Cell.
An artificial stem cell ‘niche’
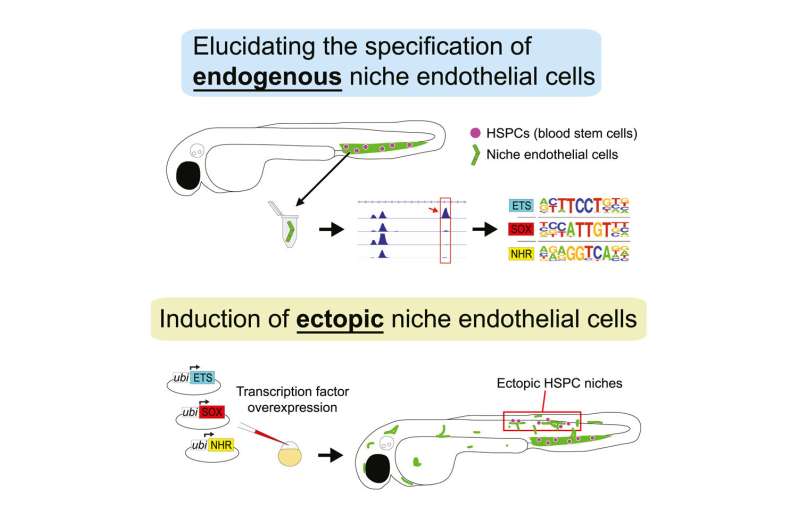
Led by Elliott Hagedorn, Ph.D., a former postdoctoral fellow in the lab, and working with zebrafish, the research team investigated the genetics of blood formation. Unlike us, zebrafish make blood in their tails. Using the technique of spatial transcriptomics, which maps gene activity in tissues, the team could see which genes were “turning on” in the tail as compared with other parts of the body.
In all, 29 different genes were strongly dialed up in the stem cell niche, especially in venous sinusoidal endothelial cells. These cells make up networks of small veins within the niche, known as venous sinusoids. Eventually, the team narrowed its investigation to three transcription factors, which bind to DNA and activate specific genes (in this case, an Ets, SoxF, and nuclear hormone receptor family member). The combination of these three transcription factors was enough to create a stem cell niche, they found.
The team then used genetic techniques to get the endothelial cells to make more of these factors. This reprogrammed the cells to become venous sinuosoids.
“When we overexpressed these factors, we could get different parts of the embryo to create blood,” says Zon, who is also part of the Division of Hematology/Oncology at Boston Children’s and Dana-Farber Cancer Institute. “We basically created a marrow in a new location.”
Hagedorn told Zon that it was the best day he’d ever had as a postdoc. The team could actually observe blood stem cells leaving their usual niche in the zebrafish tail and migrating to the new location. They even saw the cells being cuddled and dividing.
A new source of cells for blood disorders?
Zon believes the work could translate fairly readily to people. “The stem cell niche in embryonic zebrafish looks very similar to human bone marrow,” he says. “Wherever you make blood in any animal, our signature of genes is expressed.”
Though the concept sounds like science fiction, it has a lot of potential applications in disease. The ability to create new blood stem cell niches could lead to strategies to expand a person’s blood stem cells when the bone marrow is damaged or diseased, and without the need to find a bone marrow donor.
One condition Zon has in mind is myelofibrosis, in which the bone marrow niche is scarred and unsuitable for nurturing stem cells. By creating a new niche, the cells could have a “safe harbor” to live and generate blood cells. Within the niches, stem cells could be directed to make the types of blood cells that are most needed.
“Now that we can make a new niche, we can do ‘niche therapy,'” says Zon.
More information:
Elliott J. Hagedorn et al, Transcription factor induction of vascular blood stem cell niches in vivo, Developmental Cell (2023). DOI: 10.1016/j.devcel.2023.04.007
Journal information:
Developmental Cell
Source: Read Full Article


