An anti-inflammatory compound may have the potential to treat systemic inflammation and brain injury in patients with severe COVID-19 and significantly reduce their chances of death, according to a new study from UTHealth Houston and other institutions.
A team of researchers including UTHealth Houston faculty members Aaron M. Gusdon, MD, assistant professor in the Vivian L. Smith Department of Neurosurgery with McGovern Medical School at UTHealth Houston; H. Alex Choi, MD, associate professor in the department as well as the Department of Neurology; and Louise D. McCullough, MD, Ph.D., professor and Roy M. and Phyllis Gough Huffington Distinguished Chair in the Department of Neurology, conducted a multi-site, randomized, double-blind, placebo-controlled, adaptive Phase 2 trial evaluating the safety and efficacy of an anti-inflammatory compound, called OP-101, in patients with severe COVID-19. The results of the trial were published today in Science Translational Medicine.
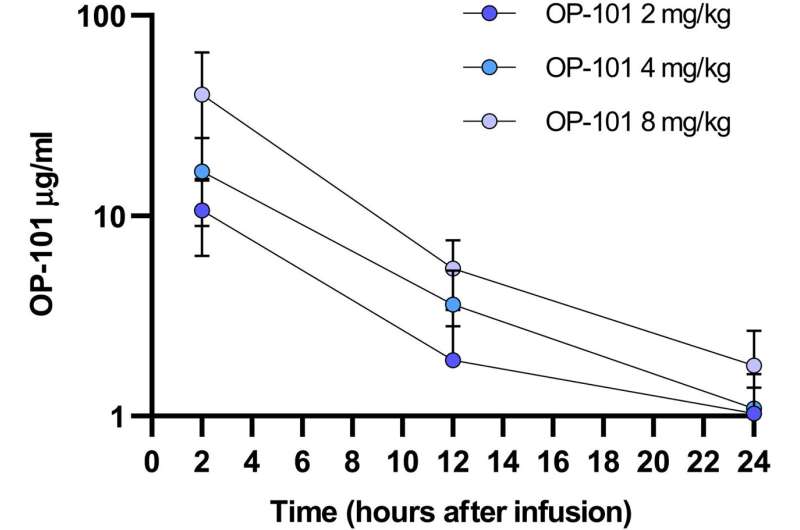
In the trial, 24 patients classified as having severe COVID-19 across five clinical sites in the U.S. were randomized to receive a single intravenous dose of placebo or OP-101 at 2, 4, or 8 mg/kg. All patients received standard of care, including corticosteroids.
“OP-101 is a novel nanotherapeutic compound that specifically targets activated macrophages and microglia, the primary immune cell in the brain,” said Gusdon, who was first author on the study. “Due to its excellent safety profile, we were excited to offer this therapy to these critically ill patients at Memorial Hermann Hospital.”
Hyperinflammation triggered by SARS-CoV-2 is a major cause of disease severity in COVID-19. OP-101 was found to be better than a placebo at decreasing inflammatory markers, as well as better at reducing markers of neurological injury, including neurofilament light chain and glial fibrillary acidic protein.
Additionally, risk for the composite outcome of mechanical ventilation or death at 30 or 60 days after treatment was 71% for patients receiving the placebo, but just 18% for patients in the pooled OP-101 treatment arms. At 60 days after treatment, 3 of 7 patients given placebo and 14 of 17 patients treated with OP-101 survived.
The data shows that OP-101 was well tolerated in the critically ill patient population and could serve as an effective treatment for patients hospitalized with COVID-19.
“Although this was a small-dose escalation trial, there was clearly a strong signal toward benefit at both acute and chronic timepoint,” Gusdon said. “The possibility that this therapy could also benefit patients with other diseases that lead to systemic inflammatory responses, including various forms of brain injury, is extremely exciting.”
OP-101 is a nanotherapeutic compound that has previously been evaluated in several animal models of inflammatory disease and has shown superior anti-inflammatory and anti-oxidant effects.
Source: Read Full Article


