A recent research paper posted to the bioRxiv* preprint server demonstrated that a cocktail of two synergetic antibodies extensively neutralized severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants, including Omicron BA.1 and BA.2.
 Study: A cocktail containing two synergetic antibodies broadly neutralizes SARS-CoV-2 and its variants including Omicron BA.1 and BA.2. Image Credit: Kateryna Kon / Shutterstock
Study: A cocktail containing two synergetic antibodies broadly neutralizes SARS-CoV-2 and its variants including Omicron BA.1 and BA.2. Image Credit: Kateryna Kon / Shutterstock
Background
The coronavirus disease 2019 (COVID-19) pandemic continues globally even after two years since its emergence. SARS-CoV-2 infections can be prevented and treated using neutralizing antibodies (NAbs). Despite this, emerging SARS-CoV-2 variants, such as Omicron, have dramatically reduced the effectiveness of the most commonly used NAbs.
Furthermore, existing reports suggest that no authorized NAbs, except the recently approved agent LY-CoV1404 (bebtelovimab), effectively target all Omicron variant sublineages. Thus, the discovery of conserved crucial SARS-CoV-2 epitopes and the selection of NAbs with extensive neutralizing capabilities are still desperately needed.
About the study
In the present study, the investigators conducted a single B-cell sorting from COVID-19 convalescent subjects to identify a NAb that widely targeted the spike (S) receptor-binding domain (RBD) protein of currently prevalent SARS-CoV-2 variants, like Delta and Omicron.
The scientists assessed the SARS-CoV-2 neutralizing capacity of 55A8 NAb and its combination with a NAb named 58G6 previously discovered by the authors. The team mixed 55A8 and 58G6 NAbs, establishing a complimentary cocktail or 2-cocktail. Further, they analyzed how well the 2-cocktail could neutralize the SARS-CoV-2 wild-type (WT), Omicron BA.2, Delta, and Omicron BA.1 pseudo and authentic viruses, except the Omicron BA.2 authentic virus.
The interactions between Omicron BA.1 S trimers and 55A8 were assessed using cryogenic electron microscopy (cryo-EM). In the 55A8 fragment antigen-binding (Fab)-BA.1 S structures and angiotensin-converting enzyme 2 (ACE2)-BA.1 S complexes, the up RBD was superimposed. The cryo-EM structures of Omicron BA.1 S trimers in a complex with 58G6 and 55A8 Fabs were determined to analyze the possibility of the 58G6 and 55A8 Fabs combination in-depth. The effects of 58G6 and 55A8 (2-cocktail) combination therapy on the Omicron infection were evaluated by in vivo and in vitro assessments.
Results
The study results revealed 55A8, an extraordinarily potent SARS-CoV-2 antibody, via single B-cell sorting from COVID-19-recovered individuals that targeted the SARS-CoV-2 S protein RBD. The authors found that 55A8 bound with SARS-CoV-2 WT and Omicron BA.2 and BA.1 strains concurrently with 58G6.
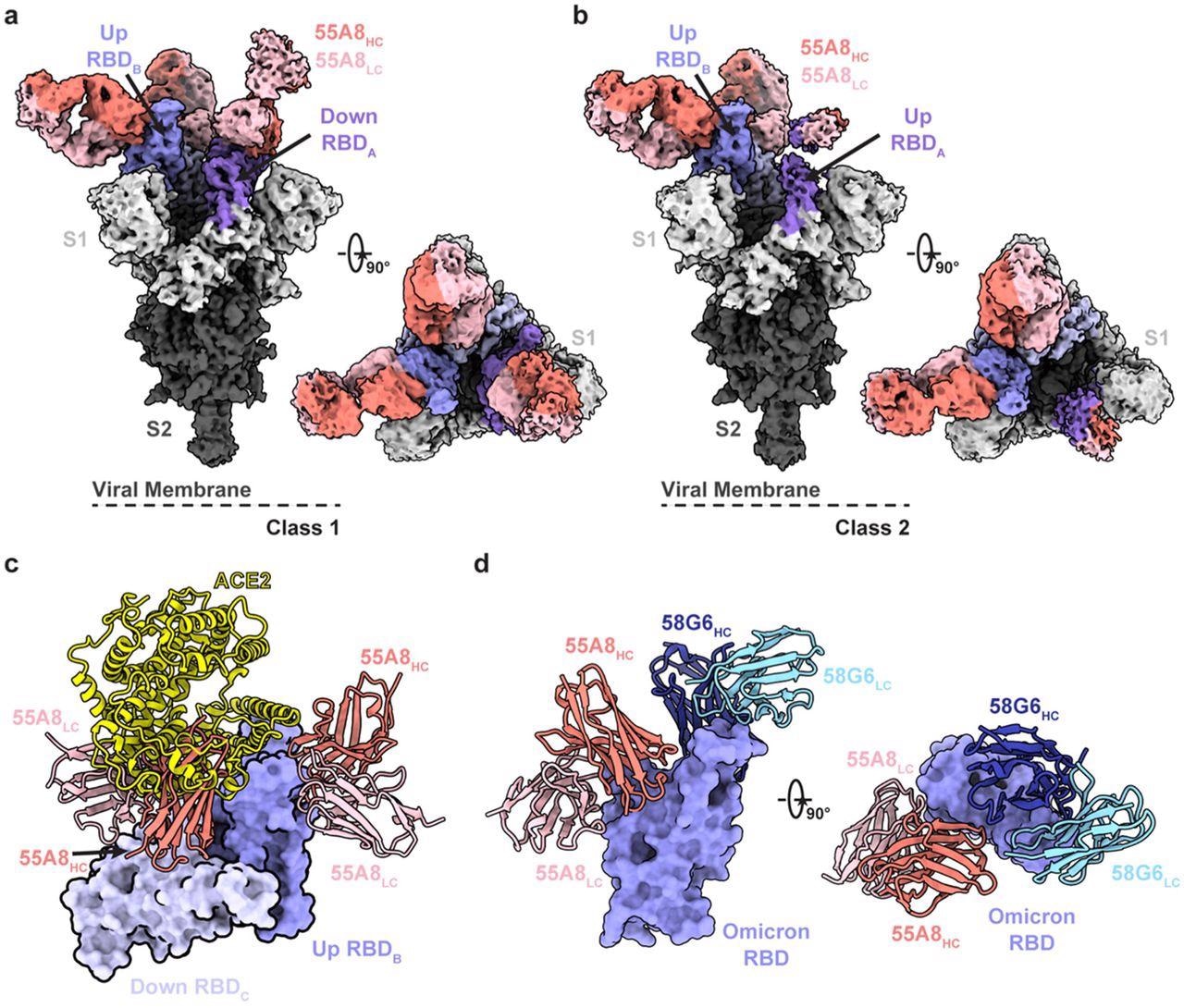
Cryo-EM structures of 55A8 binding to the SARS-CoV-2 Omicron S protein. a-b The cryo-EM densities of the 55A8 Fab-Omicron S complex we e observed in two classes (a, Class I, 3.4 Å, 3 Fabs bound with Omicron S in the “2-up/1-down” conformation; b, Class II, 3.4 Å, 3 Fabs bound with Omicron S in the “1-up/2-down” conformation). c Superposition of the locally refined Omicron RBD-ACE2 (PDB ID: 7WSA) model together with the locally refined Omicron RBD-55A8 Fab model. d Locally refined model of the 55A8 Fab and 58G6 Fab on the same up Omicron RBD. HC, heavy chain; LC, light chain.
According to the cryo-EM structural study, 55A8 was a Class III NAb that identified a highly conserved SARS-CoV-2 epitope. 55A8 might prevent ACE2 from binding to the SARS-CoV-2 S protein RBD trimer through steric hindrance.
55A8 and 58G6 NAbs demonstrated unique binding sites based on the biolayer interferometry (BLI) competition assay and cryo-EM analysis. 58G6 was primarily attached to the receptor-binding motif (RBM), whereas 55A8 identified regions located on the external surface of the RBD (S440-450 and S345-352 sites). 58G6 and 55A8 NAbs bound to non-overlapping epitopes in the SARS-CoV-2 RBD and hindered RBD interaction with ACE2 diversely. Thus, the RBD epitopes targeted by 55A8 and 58G6 were complementary and distinct, possibly explaining how these two NAbs work together.
The authors noticed that 55A8 had somewhat lower neutralizing effectiveness against the SARS-CoV-2 Lambda, Kappa, and Delta VOCs, probably due to the mutation at the L452 location. Kappa and Delta have the L452R mutation, while Lambda has the L452Q mutation. Additionally, 58G6 harbored was marginally low neutralizing capacity against the Omicron BA.2 and BA.1 sublineages. Compared to other RBM-targeted NAbs, 58G6 may form hydrogen bonds with the altered amino acids K478, R493, and N477, suggesting that this NAb has preserved neutralizing effectiveness against Omicron.
A 55A8 and 58G6 antibody cocktail (2-cocktail) demonstrated synergistic SARS-CoV-2 neutralizing efficacy in vitro, with a picomolar ranged half-maximal inhibitory concentration (IC50). Furthermore, intranasal delivery of the 58G6/55A8 antibody cocktails displayed preventive effectiveness in Omicron BA.1-challenged hamsters at an incredibly low dose of 25 g of each antibody daily at three days post-infection (dpi).
The present findings uncovered essential epitope information for the generation of improved antiviral medicines and a possible antibody cocktail for clinical usage against infection with existing SARS-CoV-2 and future mutated variants.
Conclusions
The study findings reported a novel NAb called 55A8 broadly neutralizing SARS-CoV-2 WT, Omicron BA.2, and BA.1 variants with remarkable efficacy. Furthermore, 55A8 and 58G6 demonstrated synergetic actions that increased the SARS-CoV-2 neutralizing efficiency and breadth, both in vivo and in vitro.
Structural assessments depicted that 55A8 was a Class III NAb recognizing a conserved SARS-CoV-2 epitope. As a result, a 58G6 and 55A8 cocktail, or two-cocktail, might be designed to counteract presently circulating SARS-CoV-2 VOCs, such as Omicron, and newly emerging VOCs.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- A cocktail containing two synergetic antibodies broadly neutralizes SARS-CoV-2 and its variants including Omicron BA.1 and BA.2; Xinghai Zhang, Feiyang Luo, huajun Zhang, Hangtian Guo, Junhui Zhou, Tingting Li, Shaohong Chen, Shuyi Song, Meiying Shen, Yan Wu, Yan Gao, Xiaojian Han, Yingming Wang, Chao Hu, Yuchi Lu, Wei Wang, Kai Wang, Ni Tang, Tengchuan Jin, Chengyong Yang, Guofeng Cheng, Haitao Yang, Aishun Jin, Xiaoyun Ji, Rui Gong, Sandra Chiu, Ailong Huang, bioRxiv preprint 2022, DOI: https://doi.org/10.1101/2022.04.26.489529, https://www.biorxiv.org/content/10.1101/2022.04.26.489529v1
Posted in: Drug Discovery & Pharmaceuticals | Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: ACE2, Angiotensin, Angiotensin-Converting Enzyme 2, Antibodies, Antibody, Antigen, Assay, Cell, Cell Sorting, Coronavirus, Coronavirus Disease COVID-19, covid-19, Efficacy, Electron, Electron Microscopy, Enzyme, in vitro, in vivo, Microscopy, Mutation, Omicron, Pandemic, Protein, Receptor, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Virus

Written by
Shanet Susan Alex
Shanet Susan Alex, a medical writer, based in Kerala, India, is a Doctor of Pharmacy graduate from Kerala University of Health Sciences. Her academic background is in clinical pharmacy and research, and she is passionate about medical writing. Shanet has published papers in the International Journal of Medical Science and Current Research (IJMSCR), the International Journal of Pharmacy (IJP), and the International Journal of Medical Science and Applied Research (IJMSAR). Apart from work, she enjoys listening to music and watching movies.
Source: Read Full Article