Combining a PD-1 immune checkpoint inhibitor with standard chemotherapy improved quality of life for patients with advanced stomach cancer or esophageal cancer compared to chemotherapy alone, according to recent findings published in the Journal of Clinical Oncology.
“In most oncology trials, and almost all of them prior to the introduction of modern immunotherapy, the addition of a new, experimental agent has been associated with added toxicity that often contributed to worsened (or at least not improved) quality of life relative to the standard comparator therapy. In those cases, one would have to weigh the benefit of added survival time with the risk of worse life quality due to added treatment burden. But in this case, we have a ‘win-win’ for patients,” said David Cella, Ph.D., professor of Medical Social Sciences, director of the Center for Patient-Centered Outcomes and a co-author of the study.
The average five-year survival rate for advanced stomach cancer is 7%, according to the National Cancer Institute, and current treatments may worsen symptoms and quality of life for patients. Comparatively, esophageal cancer has an average five-year survival rate of 20%, though a patient’s prognosis may slightly improve if the cancer is detected earlier.
Nivolumab, a PD-1 immune checkpoint inhibitor that has been approved by the FDA to treat both advanced stomach cancer and esophageal cancer either alone or in addition to other drugs, binds to the protein PD-1 on the surface of T-cells, preventing cancer cells from suppressing the immune system.
A previous report on the CheckMate 649 clinical trial showed that the addition of nivolumab to chemotherapy improved progression-free survival and overall survival in patients with advanced stomach cancer and esophageal cancer, prompting Cella and colleagues to further investigate patient-reported outcomes in this patient population.
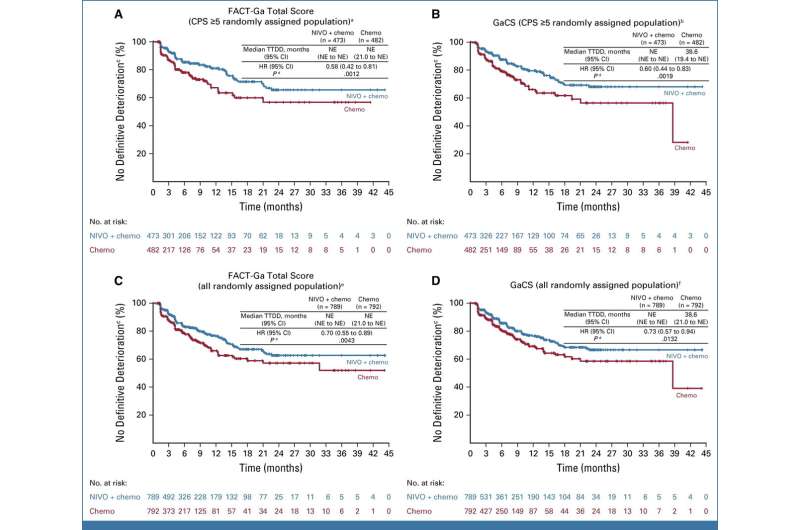
In the current study, more than 1,300 randomized patients were examined for quality of life following nivolumab plus chemotherapy treatment or chemotherapy alone. Quality of life was measured using a self-reported questionnaire developed by Cella, called the Functional Assessment of Cancer Therapy-Gastric (FACT-Ga).
Overall, patients who received immunotherapy plus chemotherapy reported better quality of life than those who received only chemotherapy. The findings complement those of the previous report, suggesting that people with the combined therapy not only lived longer, but lived better, according to Cella.
More information:
Markus Moehler et al, Health-Related Quality of Life With Nivolumab Plus Chemotherapy Versus Chemotherapy in Patients With Advanced Gastric/Gastroesophageal Junction Cancer or Esophageal Adenocarcinoma From CheckMate 649, Journal of Clinical Oncology (2023). DOI: 10.1200/JCO.23.00170
Journal information:
Journal of Clinical Oncology
Source: Read Full Article