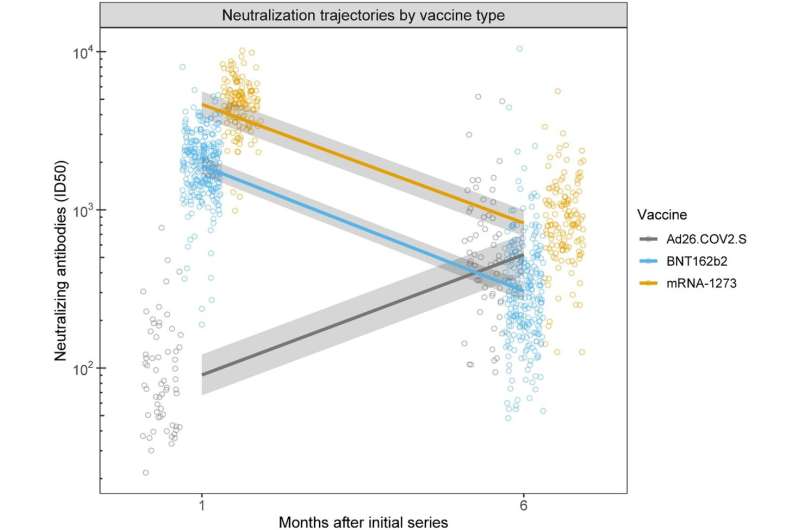
COVID vaccines offered varying degrees of protection in the six months after patients receive them, with levels climbing and plunging in two cases and climbing and climbing in another, according to new UC San Francisco research published in Scientific Reports. Age, gender, body mass index (BMI) and smoking status also play roles, according to researchers.
In the BOOST study (Building Optimal antibOdies STudy), researchers tracked the response to the Pfizer, Moderna and Johnson & Johnson vaccines in blood samples of 498 healthy volunteers, ages 18 to 88. They measured levels of neutralizing antibodies—the proteins secreted by plasma cells that block pathogens, like the COVID virus, thus preventing infection.
All three vaccines had been authorized by the Food and Drug Administration (FDA) when the study took place in spring 2021.
One month after their shots, volunteers who received the Pfizer vaccine had an antibody response 21 times higher than those who received Johnson & Johnson, while those on Moderna streaked ahead with a vaccine response 51 times higher than those on Johnson & Johnson. To ensure accuracy, volunteers were tested for prior COVID infection before getting the vaccine. Any earlier infection was accounted for in calculating antibody levels.
Surprisingly, at six months, those on Johnson & Johnson gained momentum, overtaking the antibody levels of those on Pfizer and equaling those on Moderna—as those vaccines’ antibody levels declined. “This was the most striking finding,” said first author Aric Prather, Ph.D., of the UCSF Department of Psychiatry and Behavioral Sciences, and the Weill Institute for Neurosciences. “While the mechanism for this increase is unknown, other smaller studies have suggested this trend, leading us to believe there are robust phenomena not specific to our sample.”
Smoking blunts vaccine response 240%
Confirming previous studies, the researchers found that other factors impacted effectiveness. Among older adults, antibodies as a whole were 17% lower for those on Pfizer and 11% lower for those on Johnson & Johnson but remained the same for those on Moderna; they were 11% lower for those with higher BMI on Johnson & Johnson, but not for those on Moderna or Pfizer; they were 30% lower in men than for women, irrespective of vaccine type, and they were 240% lower for smokers than for nonsmokers, irrespective of vaccine type.
“It is encouraging that the stronger vaccine, Moderna, mitigated the effects of some risk factors, particularly age,” said senior author Elissa Epel, Ph.D., vice chair of the UCSF Department of Psychiatry and Behavioral Sciences.
Both Pfizer and Moderna use RNA technology, a genetic code delivered to cells that primes the immune system to recognize and attack the coronavirus. In contrast, the Johnson & Johnson uses the more traditional virus-based technology to deliver instructions on how to defeat the virus.
The three vaccines have since been pulled from the market and in their place the FDA has recommended the newer bivalent vaccines that target the original COVID strain, as well as omicron BA.4 and BA.5, with boosters limited to seniors and those who are immunocompromised.
However, the study’s findings that certain risk factors may compromise effectiveness may hold true for the bivalents, which are also manufactured by Pfizer and Moderna. “While we have not studied the bivalent vaccination specifically, it’s likely that factors like older age, male sex, smoking status and higher BMI can prevent optimal antibody response to the vaccine,” said Epel.
The researchers’ next project will focus on the effectiveness of the Moderna and Pfizer bivalent vaccines.
More information:
Aric A. Prather et al, Predictors of long-term neutralizing antibody titers following COVID-19 vaccination by three vaccine types: the BOOST study, Scientific Reports (2023). DOI: 10.1038/s41598-023-33320-x. www.nature.com/articles/s41598-023-33320-x
Journal information:
Scientific Reports
Source: Read Full Article


