Certain fat-based molecules required for Zika virus infection in human cells could provide clues to why the virus primarily infects brain tissue, particularly in a developing fetus or newborn.
Researchers from the U.S. Department of Energy’s Pacific Northwest National Laboratory helped colleagues at Oregon Health & Science University (OHSU) identify sphingolipids as crucial to Zika virus replication in human cells. Lipids are “oily” molecules that are crucial components of many cellular structures, including cell membranes.
There is currently no treatment or vaccine for Zika virus. However, molecules that modulate cellular production of those lipids might be targets to develop a potential medicine for Zika infection, the researchers said. A paper describing the work was published July 21 in Nature Communications.
Molecular mechanism of Zika-caused disease still a mystery
In 2015 and 2016, an outbreak of Zika virus spread through North, Central and South America. Mosquitoes carry the virus and transmit it when they bite humans. Pregnant women are particularly vulnerable to Zika infection; the virus can travel to a fetus and infect nerve cells, causing congenital abnormalities and developmental delays in newborns.
To better understand what makes Zika pathogenic, researchers are working to learn everything they can about how Zika virus interacts with human cells. However, exactly how Zika virus causes disease in developing nerve cells remains a mystery.
Zika infection alters cellular lipid metabolism
In studying how particles of Zika virus interact with host cells, researchers have already identified genetic changes in Zika-infected cells and pinpointed specific proteins that the virus uses to replicate itself.
However, researchers are still learning about how Zika infection alters the molecular composition of cells. Lipids are components of particular interest in this case because they are a diverse collection of molecules that make up cell membranes. Some viruses are unable to make all the lipids they need for replication, so they also gather lipids from host cells.
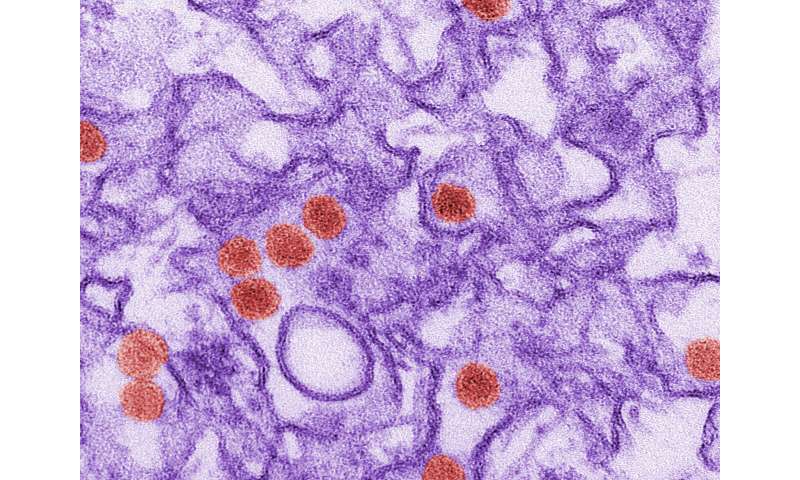
Fikadu Tafesse, a microbiologist at OHSU, thought that studying lipids involved with Zika infection might also provide more information about why the virus infects brain tissue. Compared to other organs, the brain has a high lipid content, as lipid-rich coverings around nerve cells help signals travel quickly around the brain. Perhaps the virus infects nerve cells because key molecular components for its replication are there?
To learn how a cell’s lipid components changed during Zika infection, Tafesse infected human liver cells with Zika virus and contacted colleagues at PNNL to analyze lipids extracted from the cells. The PNNL researchers, led by biomedical scientist Jennifer Kyle, used mass spectrometry to measure 340 different lipids from infected and uninfected cells. They noticed changes in several different types of lipids extracted 24 or 48 hours of infection, indicating that the infection altered cellular lipid production. None of the samples that the PNNL researchers handled contained the virus.
Sphingolipids stand out in lipidomics analysis
Then the researchers analyzed lipids extracted from human liver cells exposed to only one protein from the Zika virus. They noticed similar changes in lipid patterns to samples from cells infected with the entire virus. Interestingly only one type of lipid, ceramide, was elevated in both experiments.
Ceramide is part of a class of lipids called sphingolipids. When the researchers treated human liver and brain cells with a molecule that blocks cellular production of sphingolipids, they saw significantly reduced amount of virus replication. From those results, the researchers concluded that sphingolipids are required for viral replication. And with further experiments, they pinpointed a particular step in the cellular process of sphingolipid production that influenced viral replication.
“By mapping the wiring diagram of connections between viral replication and sphingolipid metabolism in human cells, we can identify crucial places in cellular lipid production lines that could be exploited to prevent Zika virus infection,” Tafesse said.
Targets for potential drug development, once identified, include viral proteins that induce sphingolipid production in human cells. Human proteins involved in sphingolipid production might be a candidate for drug development too, if a drug can be made to slow viral replication without harming regular human metabolism too much, he said.
This work is part of an ongoing collaboration between PNNL and OHSU called the Precision Medicine Innovation Co-Laboratory, or PMedIC. In this collaboration, researchers combine information about large-scale patterns in genes, proteins, and small molecules in cells with information from medical imaging. The result is a multi-faceted understanding of disease from the molecular to a medical level. This understanding can help medical scientists better understand how disease happens or develop customized treatments.
Source: Read Full Article