Over the last decade, advances in 3D printing have unlocked new possibilities for bioengineers to build heart tissues and structures. Their goals include creating better in vitro platforms for discovering new therapeutics for heart disease, the leading cause of death in the United States, responsible for about one in every five deaths nationally, and using 3D-printed cardiac tissues to evaluate which treatments might work best in individual patients.
A more distant aim is to fabricate implantable tissues that can heal or replace faulty or diseased structures inside a patient’s heart.
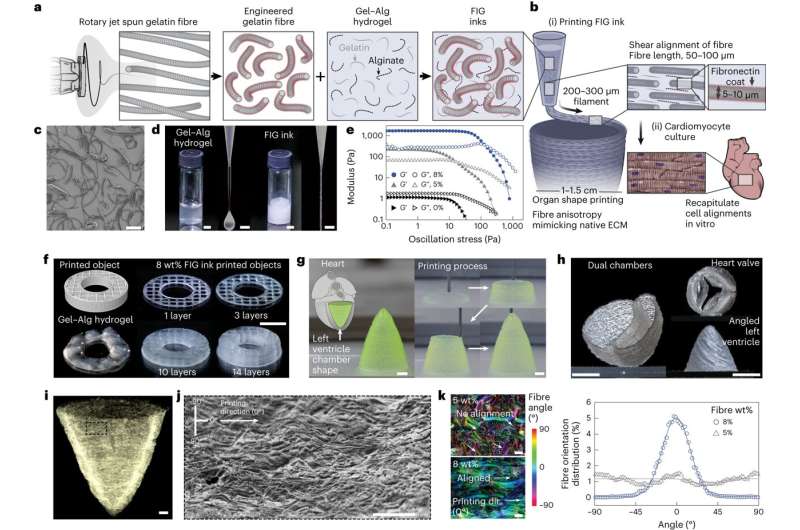
In a paper published in Nature Materials. researchers from the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS) report the development of a new hydrogel ink infused with gelatin fibers that enables 3D printing of a functional heart ventricle that mimics beating like a human heart. They discovered the fiber-infused gel (FIG) ink allows heart muscle cells printed in the shape of a ventricle to align and beat in coordination like a human heart chamber.
“People have been trying to replicate organ structures and functions to test drug safety and efficacy as a way of predicting what might happen in the clinical setting,” says Suji Choi, research associate at SEAS and first author on the paper. But until now, 3D printing techniques alone have not been able to achieve physiologically-relevant alignment of cardiomyocytes, the cells responsible for transmitting electrical signals in a coordinated fashion to contract heart muscle.
“We started this project to address some of the inadequacies in 3D printing of biological tissues,” says Kevin “Kit” Parker, Tarr Family Professor of Bioengineering and Applied Physics and head of the Disease Biophysics Group at SEAS, and senior author.
The innovation lies in the addition of fibers within a printable ink. “FIG ink is capable of flowing through the printing nozzle but, once the structure is printed, it maintains its 3D shape,” says Choi. “Because of those properties, I found it’s possible to print a ventricle-like structure and other complex 3D shapes without using extra support materials or scaffolds.”
To create the FIG ink, Choi leveraged a rotary jet spinning technique developed by Parker’s lab that fabricates microfiber materials using an approach similar to the way cotton candy is spun. Postdoctoral researcher Luke MacQueen, a co-author on the paper, proposed the idea that fibers created by the rotary jet spinning technique could be added to an ink and 3D printed.
“When Luke developed this concept, the vision was to broaden the range of spatial scales that could be printed with 3D printers by dropping the bottom out of the lower limits, taking it down to the nanometer scale,” Parker says. “The advantage of producing the fibers with rotary jet spinning rather than electrospinning”—a more conventional method for generating ultrathin fibers—”is that we can use proteins that would otherwise be degraded by the electrical fields in electrospinning.”
Using the rotary jet to spin gelatin fibers, Choi produced a sheet of material with a similar appearance to cotton. Next, she used sonification—sound waves—to break that sheet into fibers about 80 to 100 micrometers long and about 5 to 10 micrometers in diameter. Then, she dispersed those fibers into a hydrogel ink.
“This concept is broadly applicable—we can use our fiber-spinning technique to reliably produce fibers in the lengths and shapes we want,” Choi says. The most difficult aspect was troubleshooting the desired ratio between fibers and hydrogel in the ink to maintain fiber alignment and the overall integrity of the 3D-printed structure.
As Choi printed 2D and 3D structures using FIG ink, the cardiomyocytes lined up in tandem with the direction of the fibers inside the ink. By controlling the printing direction, Choi could therefore control how the heart muscle cells would align.
When she applied electrical stimulation to 3D-printed structures made with FIG ink, she found it triggered a coordinated wave of contractions in alignment with the direction of those fibers. In a ventricle-shaped structure, “it was very exciting to see the chamber actually pumping in a similar way to how real heart ventricles pump,” Choi says.
As she experimented with more printing directions and ink formulas, she found she could generate even stronger contractions within ventricle-like shapes.
“Compared to the real heart, our ventricle model is simplified and miniaturized,” she says. The team is now working toward building more life-like heart tissues with thicker muscle walls that can pump fluid more strongly. Despite not being as strong as real heart tissue, the 3D-printed ventricle could pump 5-20 times more fluid volume than previous 3D-printed heart chambers.
The team says the technique can also be used to build heart valves, dual-chambered miniature hearts, and more.
“FIGs are but one tool we have developed for additive manufacturing,” Parker says. “We have other methods in development as we continue our quest to build human tissues for regenerative therapeutics. The goal is not to be tool driven—we are tool agnostic in our search for a better way to build biology.”
More information:
Choi, S. et al. Fibre-infused gel scaffolds guide cardiomyocyte alignment in 3D-printed ventricles. Nature Materials (2023). DOI: 10.1038/s41563-023-01611-3. www.nature.com/articles/s41563-023-01611-3
Journal information:
Nature Materials
Source: Read Full Article


