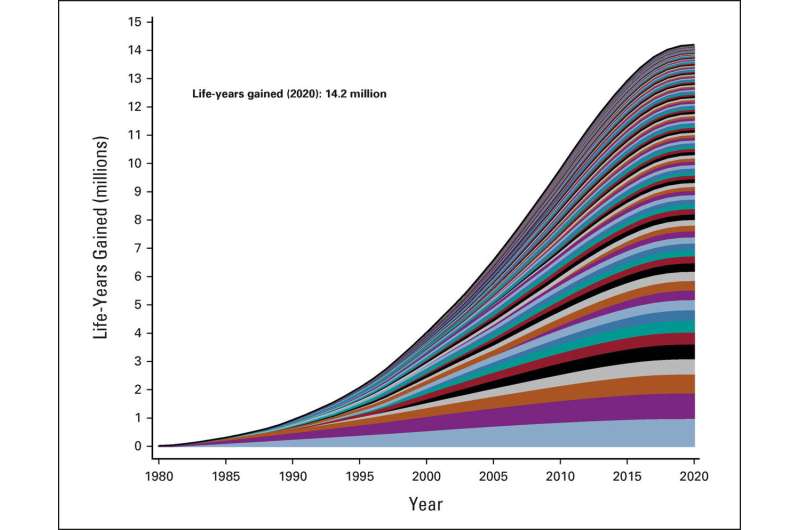
Clinical trials in adults conducted within the National Cancer Institute’s (NCI’s) National Clinical Trials Network (NCTN) over the last four decades are estimated to have extended the lives of patients with cancer in the U.S. by at least 14.2 million life-years, according to a new analysis. Importantly, the study estimated that the costs of those gains have been quite modest: roughly $326 in federal investment for each life-year added.
The results are published in the Journal of Clinical Oncology. The analysis was led by researchers from the SWOG Cancer Research Network, one of the NCTN clinical trials groups funded by the NCI, which is part of the National Institutes of Health (NIH).
The research team also quantified some of the enormous clinical and scientific impact the results of NCTN trials have had. Overall, the researchers identified 162 phase III trials conducted since 1980 with positive results. Primary findings of the 162 analyzed studies were cited in the scientific literature more than 165,000 times through the end of 2020, and 87.7% of the trials have been referred to as evidence in subsequent cancer care recommendations made in widely used guidelines.
Joseph M. Unger, Ph.D., a SWOG health services researcher and biostatistician with Fred Hutchinson Cancer Center, is lead author on the work.
“This work provides a clear statement about the impact of publicly funded cancer research,” Dr. Unger said. “When we discuss investments in infrastructure, it’s important to also consider scientific infrastructure. In the U.S., the NCI-sponsored network groups have been a vital element of the scientific infrastructure of clinical research for decades, and have returned incredible value to cancer patients and to the U.S. taxpayer.”
To estimate the life-years gained from NCTN research, the team looked at trials conducted by the four adult network groups (or their predecessors): the Alliance for Clinical Trials in Oncology, the ECOG-ACRIN Cancer Research Group, NRG Oncology, and SWOG Cancer Research Network. Together with the Children’s Oncology Group, which performs research into childhood cancers, these groups are the core of a network that, in conjunction with the NCI Community Oncology Research Program (NCORP), runs clinical trials at more than 2,200 academic and community treatment sites across the U.S. and beyond, and they have conducted publicly funded research into effective new cancer treatments for more than half a century.
“First and foremost, we commend the generosity and courage of patients who participate in clinical trials,” said Monica M. Bertagnolli, M.D., director of NCI. “This work is a clear demonstration that those who do so make a tremendous contribution to society. We also acknowledge the work of clinical providers and other caregivers who go above and beyond usual care to make clinical trials possible. This result validates the key role of NCTN and other networks in improving the outlook for patients with cancer and progressing toward our goal to reduce the cancer death rate by half within 25 years.”
For the current analysis of the work of the adult NCTN groups, researchers identified randomized phase 3 studies with primary results published in or after 1980 that reported an improvement in a time-dependent outcome favoring the experimental treatment. The 162 trials they analyzed had enrolled at total of 108,334 patients.
For each trial showing an overall survival benefit on the experimental arm, the researchers mapped the benefit onto the U.S. cancer population using national cancer registry and life table data.
The 14.2 million life-years these trials added through 2020 represents 4.2% of the 336.8 million years of life lost in the U.S. due to cancer from 1980–2020. The researchers further project that by 2030, this same set of already completed studies will have added 24.1 million life-years for patients.
Total federal investment to conduct these trials was calculated using public data on estimated funding for the four NCTN groups—a total of $4.63 billion in federal investment over those 40 years. To put this $4.63 billion figure (an average yearly investment of about $116 million) in context, in the year 2015 alone, the U.S. spent $183 billion on cancer care, and the total years of life lost to 2015 adult cancer deaths in the U.S. resulted in an estimated $94.4 billion loss in future earnings.
The impact of the results on clinical care was measured by determining whether a trial’s results were cited as evidence in favor of a recommended treatment in major clinical guidelines or in package inserts for new drug approvals by the U.S. Food and Drug Administration. Overall, the findings from 87.7% of the trials were used to support recommended clinical care. The overall scientific impact of the trial findings was assessed by determining how often the primary report on each study’s results was cited in Google Scholar. In total, the 162 trials were cited 165,336 times through 2020, and nearly all of the trials (90.1%) were published in high-impact scientific journals.
The authors acknowledge that the $326 per life-year gained federal investment figure does not encompass all costs, noting that the costs of initial drug discovery and early testing are not included in the analysis, nor are the full costs of conducting these trials, including the costs borne by clinical institutions and the time and effort of research investigators.
On the other hand, the authors also discuss multiple additional benefits patients have derived from these and other NCTN clinical trials that are not accounted for in these statistics.
“These trials have also made substantial contributions in ways that are harder to measure,” Dr. Unger added. “For instance, negative trials are critical for showing what treatments should not be used, and federally sponsored research groups also provide critical opportunities to mentor new generations of clinical researchers. And for patients, these trials routinely provide access to treatments for underserved patient populations that may not be as accessible in industry-sponsored trials.”
More information:
Joseph M. Unger et al, Population, Clinical, and Scientific Impact of National Cancer Institute’s National Clinical Trials Network Treatment Studies, Journal of Clinical Oncology (2022). DOI: 10.1200/JCO.22.01826
Journal information:
Journal of Clinical Oncology
Source: Read Full Article