NOTICE: This Consumer Medicine Information (CMI) is intended for persons living in Australia.
FULPHILA
contains the active ingredient pegfilgrastim (rbe)
CONSUMER MEDICINE INFORMATION
What is in this leaflet
This leaflet answers some common questions about FULPHILA.
It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor has prescribed FULPHILA after considering its likely benefit to you, as well as the potential risks.
If you have any concerns about taking this medicine, talk to your doctor, nurse or pharmacist.
Keep this leaflet with your medicine.
You may need to read this information again.
What this medicine is used for
FULPHILA is used following chemotherapy to help fight infection.
Some chemotherapy will reduce the number of neutrophils in your body. Although FULPHILA is not a treatment for cancer, it does help the body to make new neutrophils. This will reduce your chance of developing infections that might require antibiotics and/or hospital stays. It may even increase your chance of receiving your chemotherapy on time and at the right dose.
How it works
Fulphila is a long acting form of Recombinant Human Granulocyte Colony Stimulating Factor or G-CSF. Using gene technology, Fulphila is produced in a specific type of bacteria, called E. coli.
G-CSF is produced in the bone marrow and assists in the production of neutrophils, which are a type of white blood cell. Neutrophils help the body fight infections by surrounding and destroying the bacteria that cause the infections.
G-CSF also helps neutrophils to do this work better.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
This medicine is available only with a doctor’s prescription.
Before you use it
When you must not use it
Do not have FULPHILA if you have an allergy to:
Any medicine containing pegfilgrastim or filgrastim
Any of the ingredients listed at the end of this leaflet
Any medicines or products that are products that are produced using the bacteria E.coli.
Symptoms of an allergic reaction may include:
shortness of breath, wheezing or difficulty breathing
swelling of the face, lips, tongue or other parts of the body
skin rash, itching or hives.
Do not use FULPHILA at the same time as your chemotherapy or radiotherapy.
Do not use FULPHILA within 24 hours after you receive chemotherapy.
This is because the chemotherapy medicine may stop FULPHILA from increasing the number of infection fighting neutrophils.
Do not use FULPHILA after the expiry date (EXP) printed on the pack.
Do not use FULPHILA if the packaging is torn or shows signs of tampering.
Do not use FULPHILA if it has been left out of the refrigerator.
If you are not sure whether you should use FULPHILA, talk to your doctor or pharmacist.
Before you start to use it
Tell your doctor if:
1.you have allergies to:
any other medicines
any other substances, such as foods, preservatives or dyes.
2.you are pregnant or intend to become pregnant.
Your doctor will discuss the possible risks and benefits of having FULPHILA during pregnancy.
3.you are breastfeeding or planning to breastfeed.
Your doctor will discuss the possible risks and benefits of having FULPHILA during breastfeeding.
4.you have, or have had:
a medical condition affecting the bone marrow or blood
a family history of rare hereditary problems of fructose intolerance. This product contains sorbitol and may not suitable in such patients
sickle cell disease
problems with your kidneys, liver, heart or other organs
previous treatment for cancer
any infections, cancers or tumours.
If you have not told your doctor about any of the above, tell them before you use FULPHILA.
Taking other medicines
Tell your doctor if you are taking any other medicines, particularly those that may affect the blood. Also tell them about any medicines you buy without a prescription from your pharmacy, supermarket or health food shop.
How to use it
FULPHILA is given by injection, into the tissues just below the skin. This is called a subcutaneous injection.
Your doctor, nurse or pharmacist may suggest that you or your carer be taught how to give a subcutaneous injection. This will allow you to have your FULPHILA injection at home.
Carefully follow all directions given to you by your doctor, pharmacist or nurse. They may differ from the information in this leaflet.
If you do not understand the instructions, ask your doctor, pharmacist or nurse for help.
How to inject FULPHILA using a pre-filled syringe with an automatic needle guard
Important
Before you use FULPHILA pre-filled syringe with automatic needle guard, read this important information:
It is important that you do not try to give yourself the injection unless you have received training from your doctor or healthcare provider.
FULPHILA is given as an injection into the tissue just under the skin (subcutaneous injection).
DO NOT remove the grey needle cap from the pre-filled syringe until you are ready to inject.
DO NOT use the pre-filled syringe if it has been dropped on a hard surface. Use a new prefilled syringe and call your doctor or healthcare provider.
DO NOT attempt to activate the pre-filled syringe prior to injection.
DO NOT attempt to remove the clear pre-filled syringe safety guard from the pre-filled syringe.
DO NOT attempt to remove the peelable label on the pre-filled syringe barrel before administering your injection.
Talk to your doctor or healthcare provider if you have any questions.
Guide to parts
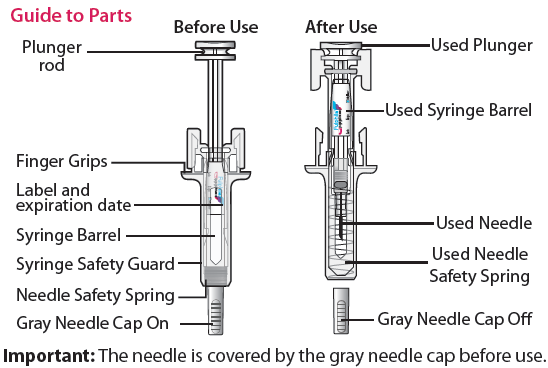
Things to do before you inject
A. Remove the pre-filled syringe tray from the package and gather the supplies needed for your injection: alcohol wipes, a cotton ball or gauze pad, an adhesive bandage and a sharps disposal container (maybe provided).
For a more comfortable injection, leave the pre-filled syringe at room temperature for about 30 minutes before injecting. Wash your hands thoroughly with soap and water.
On a clean, well-lit work surface, place the new pre-filled syringe and the other supplies.
DO NOT try to warm the syringe by using a heat source such as hot water or microwave
DO NOT leave the pre-filled syringe exposed to direct sunlight
DO NOT shake the pre-filled syringe
Keep pre-filled syringes out of the sight and reach of children
B. Open the tray, peeling away the cover. Grab the pre-filled syringe safety guard to remove the pre-filled syringe from the tray.
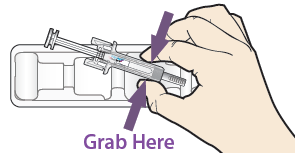
For safety reasons:
DO NOT grasp the plunger
DO NOT grasp the grey needle cap
C. Inspect the medicine and prefilled syringe.
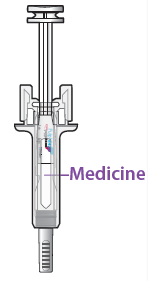
DO NOT use the pre-filled syringe if:
The medicine is cloudy or there are particles in it. It must be a clear and colourless liquid.
Any part appears cracked or broken.
The grey needle cap is missing or not securely attached.
The expiry date printed on the label has passed the last day of the month shown.
In all cases, call your doctor or healthcare provider.
Where to inject
A. Wash hands thoroughly. Prepare and clean your injection site.
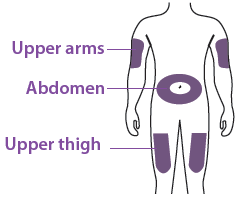
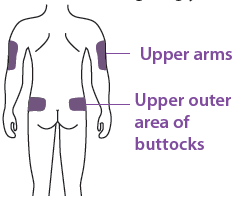
You can use:
Upper part of your thigh
Belly, except for a 5 cm (2-inch) area right around your belly butt on Outer area of upper arm (only if someone else is giving you the injection)
Clean the injection site with an alcohol wipe. Let your skin dry.
DO NOT touch the injection site before injecting
DO NOT inject into areas where the skin is tender, bruised, red, or hard. Avoid injecting into areas with scars or stretch marks.
B. Hold the prefilled syringe by the safety guard. Carefully pull the grey needle cap straight out and away from your body.
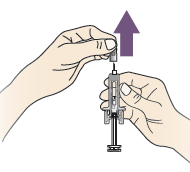
Do not twist or bend the grey needle cap.
Do not hold the prefilled syringe by the plunger rod.
Do not put the grey needle cap back onto the prefilled syringe.
C. Pinch your injection site to create a firm surface.
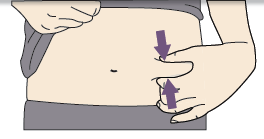
It is important to keep the ski pinched when injecting.
How to inject
A. Hold the pinch. INSERT needle into skin.
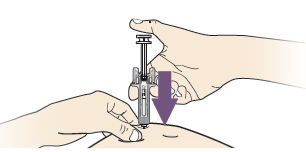
DO NOT touch the cleaned area of the skin
B. PUSH the plunger with slow and constant pressure until it reaches the bottom.
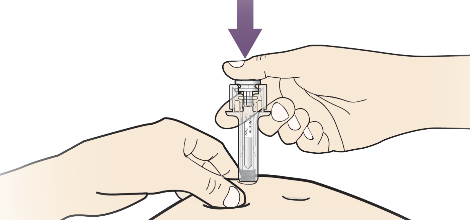
It is important that the plunger be pushed fully in order to administer your full dose.
C. RELEASE your thumb. Then LIFT the syringe off skin.
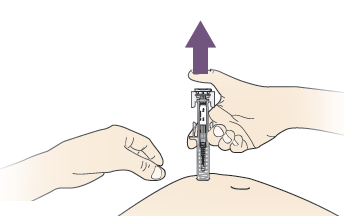
After releasing the plunger, the prefilled syringe safety guard will safely cover the injection needle.
If the guard is not activated or only partially activated, discard the product. DO NOT put the grey needle cap back on used pre-filled syringes.
When you remove the syringe, if it looks like the medicine is still in the syringe barrel, this means you have not received the full dose. Call your healthcare provider right away.
If your injection is given by another person, he or she should also be careful when removing the needle from your skin in order to prevent accidental needle stick injury and possible infections.
How to remove detachable label
Healthcare providers only
The trade name of the administered product should be clearly recorded in the patient file.
Turn the plunger to move the label into a position where you can remove the syringe label.
Remove and save the prefilled syringe label.
Disposing
A. Discard the used pre-filled syringe and other supplies in a sharps disposal container.
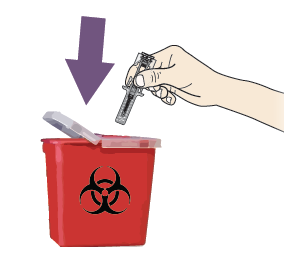
Medicines should be disposed of in accordance with local requirements. Ask your pharmacist how to dispose of medicines no longer required.
These measures will help to protect the environment.
Keep the syringe and sharps disposal container out of sight and reach of children.
DO NOT reuse the pre-filled syringe
DO NOT recycle pre-filled syringes or throw them into household waste
B. Examine the injection site.
If there is blood, press a cotton ball or gauze pad on your injection site. DO NOT rub the injection site. Apply an adhesive bandage if needed.
Further information on use
How much to inject
The usual dose is one subcutaneous injection 24 hours after the end of each chemotherapy cycle.
When to inject
Use FULPHILA 24 hours after the end of each chemotherapy cycle.
Your doctor will tell you when to begin your treatment and when to stop.
If you forget your injection
If you miss your scheduled dose, talk to your doctor, nurse or pharmacist as soon as possible.
If you inject too much (overdose)
If you inject more FULPHILA than you need, talk to your doctor, nurse or pharmacist.
If you feel unwell in any way you talk to your doctor, nurse or pharmacist immediately.
While you are using it
Things you must do
Watch for any signs or symptoms of infection.
There are many ways an infection may show itself.
Symptoms of an infection include:
fever (a temperature of 38.2°C or greater, or as your doctor suggests)
chills
rash
sore throat
diarrhoea
earache
difficult or painful breathing, coughing or wheezing
Go straight to your hospital if you develop any of these symptoms.
If you are about to be started on any new medicine, tell your doctor, nurse and pharmacist that you are using FULPHILA.
Tell any other doctors, dentists and pharmacists who treat you that you are taking this medicine.
Tell your doctor immediately if you become pregnant while taking this medicine.
Keep all of your doctor’s appointments so that your health can be monitored.
Your doctor may order blood tests to check the levels of infection-fighting neutrophils and other blood cells.
Things you must not do
Do not use FULPHILA to treat any other complaints unless your doctor tells you to.
Do not give FULPHILA to anyone else, even if they have the same condition as you.
Side effects
Tell your doctor, nurse or pharmacist as soon as possible if you have any problems while using FULPHILA, even if you do not think the problems are connected with the medicine or are not listed in this leaflet.
All medicines can have side effects. Some side effects may be serious and need medical attention. Other side effects are minor and are likely to be temporary.
You may also experience side effects caused by other medicines you are taking at the same time as FULPHILA.
Your doctor has weighed the risks of using this medicine against the benefits they expect it will have for you.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Ask your doctor, nurse or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
temporary bone pain, such as in the lower back or in the long bones of the arms or legs
This pain is usually relieved with non-prescription painkillers, like paracetamol. If you continue to have bone pain even after having taken this form of pain relief, you should speak to your doctor, as you may need a prescription medicine.
headache
general aches and pains in joints and muscles
reddish or purplish blotches under the skin
injection site pain and redness of the skin at the injection site
Tell your doctor immediately if you notice any of the following:
pain in the upper left side of the stomach (abdomen)
left shoulder pain
dizziness
fever and painful skin lesions most commonly on your arms, legs and sometimes on your face and neck
blood in the urine.
The above list includes serious side effects that may require medical attention.
Tell your doctor immediately, or go to Accident and Emergency at your nearest hospital if you notice any of the following:
swelling or puffiness
less frequent urination
swelling of your stomach-area (abdomen) and feeling of fullness
general feeling of tiredness.
These may be serious side effects of FULPHILA. You may need urgent medical attention.
Serious side effects are rare or uncommon.
If any of the following happen, stop taking FULPHILA and go straight to hospital, as you may need urgent medical attention:
rash over a large area of the body, itching or hives
shortness of breath, wheezing or difficulty breathing
swelling of the face, lips, tongue or other parts of the body
faintness
rapid pulse or sweating.
These are very serious side effects. If you have them you may have had a serious allergic reaction to FULPHILA. You may need urgent medical attention or hospitalisation.
Tell your doctor if you notice anything that worries you or that is making you feel unwell.
Other side effects not listed above may occur in some people.
After using it
Storage
Keep FULPHILA in a refrigerator at a temperature of 2°C to 8°C.
Do not freeze. Protect from light.
Do not use FULPHILA if you think it has been frozen.
Keep your medicine in its pack. Protect it from light.
Keep it where children cannot reach it.
Disposal
Once you have injected FULPHILA, do not put the grey needle cap back on the used syringe.
Discard the used syringe into an approved, puncture-resistant sharps container and keep it out of the reach of children.
Never put the used syringes into your normal household rubbish bin.
Dispose of the full puncture resistant sharps container as instructed by your doctor, nurse or pharmacist.
Product description
What it looks like
FULPHILA is a clear, colourless solution. It is supplied in a carton as a pre-filled syringe with an automatic needle guard.
Ingredients
The active ingredient in FULPHILA is 6 mg pegfilgrastim (rbe).
FULPHILA also contains:
sodium acetate
sorbitol
polysorbate 20
Water for Injections.
The grey needle cap on the pre-filled syringe with an automatic needle guard contains a derivative of latex.
FULPHILA does not contain lactose, gluten, tartrazine or any other azo dyes.
Sponsor
Alphapharm Pty Ltd
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
Phone: (02) 9298 3999
www.mylan.com.au
Australian Registration Numbers:
AUST R 282830
Date of preparation
This leaflet was prepared in 17 August 2018.
Source: Read Full Article


