They are only 10 cm long but could well be the line separating life from death in malaria. By taking the lead in iron recycling, the kidneys stop our body from surrendering to the invading parasite, Gulbenkian researchers reveal. Published in Cell Reports, this discovery has important implications for the prognosis of infected patients and for the development of targeted therapeutic strategies, which could put the brakes on the number of deaths from malaria.
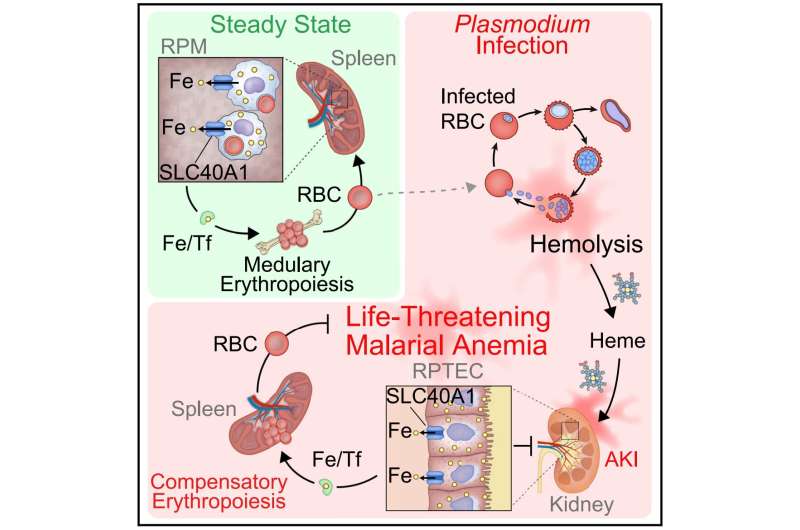
Severe and often lethal outcomes of malaria, such as acute kidney injury, emerge as the Plasmodium parasite, transmitted through mosquito bites, invades the host’s red blood cells. As the parasite multiplies exponentially, it destroys these cells and causes anemia, weakening the host’s health. In normal conditions, macrophages, a specialized cell of the immune system, recycle the iron released from damaged or old red blood cells to form new ones.
But the capacity of this system is quickly exhausted when there is mass destruction, as in malaria. In the impossibility of replacing red blood cells, the patient develops severe anemia and has a higher risk of dying.
Findings from the Instituto Gulbenkian de Ciência (IGC) show that when acute kidney injury and anemia occur simultaneously, they act synergistically to substantially increase the patient’s risk of death. These conclusions come straight from the Josina Machel-Maria Pia Hospital, in Angola, where malaria is a leading cause of death.
400 people hospitalized with malaria were included in this study stemming from a collaboration with Euclides Sacomboio, an Angolan researcher who joined the Inflammation group at the IGC through Gulbenkians ENVOLVE Ciência PALOP program.
The laboratory, led by Miguel Soares, has been studying iron metabolism in animal models of malaria for years. “It is easy to understand, through its color, that the mice we study get rid of the byproducts of red blood cells’ destruction through urine,” says Susana Ramos, the researcher who inspired and co-led the group’s most recent study.
“We began to realize that these animals also had lots of iron in their urine, but they could not possibly be getting rid of it all, even more so because these sick mice do not eat enough to sustain the needed levels of this essential micronutrient. So, we thought there must be a population of kidney cells that reabsorbs and recycles iron, to maintain some degree of normality in these anemic mice,” she explains.
In a previous study, these cells, known as renal proximal tubule cells, had already proven their importance in getting rid of toxic byproducts that promote severe forms of malaria.
Once again, this cell population proved their incredible role in preventing death from severe forms of malaria. Qian Wu, the first author of the study, found that these kidney cells alter their genetic program so that they can absorb and store iron. Later, they put the iron back in circulation, allowing for new red blood cells to form. “We were not expecting kidneys to have such an immediate role in re-establishing the development of these blood cells,” Qian says.
By becoming the main organ responsible for storing and redistributing iron during malaria, the kidneys determine the outcome of this disease. To understand the implications of this mechanism, the institute’s Transgenics Unit created mice without ferroportin, the gene that allows kidney cells to export iron. The results were surprising: the animals developed severe anemia and died, a clear example of how a simple cellular mechanism can have profound effects on the whole body.
By re-establishing the circuit of iron and the number of red blood cells, the kidneys put a brake on anemia, ensuring that the different organs receive oxygen and continue to function when the host is infected. “This finding is a clear demonstration of how the metabolism of an infected host can be re-wired to determine the outcome of an infection, in this case, malaria,” explains the principal investigator Miguel Soares.
These findings can be important to provide better personalized prognosis to infected patients. “People with some genetic mutations in this cellular iron exporter are less prone to develop severe anemia,” Miguel says. On the other hand, if the kidney cells cannot export the iron they absorb, the patients might become more susceptible to developing acute kidney injury and anemia. Now, researchers can also start thinking about therapeutic strategies directed to this metabolic pathway.
More information:
Qian Wu et al, Renal control of life-threatening malarial anemia, Cell Reports (2023). DOI: 10.1016/j.celrep.2023.112057
Journal information:
Cell Reports
Source: Read Full Article


