Our bodies are made up of 60,000 miles of complex pipes that play a vital role in transporting nutrients throughout our bodies, performing waste disposal, and supplying our organs with fresh oxygen and blood.
Vascular malformations (VMs) are a group of rare genetic disorders that causes an abnormal formation of veins, arteries, capillaries, or lymphatic vessels at birth. VMs can interfere with the duties of our precious pipes by causing blockages, poor drainage, and the formation of cysts and tangles.
To address a need for further study, William Polacheck, Ph.D., an assistant professor at the UNC-NCSU Joint Department of Biomedical Engineering and the Department of Cell Biology and Physiology, and his team spanning across the UNC School of Medicine, have developed a model that mimics VMs that are specifically caused by a mutation of PIK3CA—a gene that has been implicated in multiple types of lymphatic, capillary, and venous malformations.
Their work was published in Science Advances.
“There are number of ‘chicken and the egg problems’ of the PIK3CA mutation,” said Polacheck. “Is it causing something else to go wrong? Or is there something else in the environment that’s causing the mutation to have more severe effects? Working in a much more controlled environment, such as a microfluidic model, allows us to isolate and figure out how the genetics of the disease relate to what is happening in the cells.”
VMs are caused by mutations in the genes that directs the development of the vasculature throughout the body. Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) is one of those genes. It is a metaphorical “hotspot” for mutations that contribute to malformations of the smaller blood vessels, causing blood to pool underneath the skin.
This specific type of vascular malformation is less studied than others and it is usually discovered at birth. These diseases start as the baby is developing. Since there are a multitude of changes happening at this point in the child’s development, it can be difficult condition for researchers to study.
A Team in the Making
Julie Blatt, MD, a professor of pediatric hematology-oncology in the UNC Department of Pediatrics, saw a need for a new approach to model the disease, which affects a majority of her patients.
Dr. Blatt, who has an interest in repurposing cancer drugs to treat pediatric patients with vascular anomalies, picked up the phone and cold-called Polacheck, who is a biomedical engineer by trade, to ask if he could create a microfluidic model of PIK3CA0-specific vascular malformations.
Around the same time, Wen Yih Aw, Ph.D., was working on her postdoctoral degree at UNC Catalyst, a research group focused on understanding rare diseases in the Eshelman School of Pharmacy. Eventually, Aw started using her basic science expertise with the Polacheck lab on a different project.
In addition to Blatt and Aw, the lab has collaboration with Boyce Griffith, Ph.D. in the Department of Mathematics and the Computational Medicine Program, who is helping with analyzing the structures of the networks.
“All those pieces, I think were necessary to complete the work,” said Polacheck. “It does say something about UNC because there were multiple colleges and departments working together. There were no barriers whatsoever to working together on this project.”
How a Microfluidic Model Works
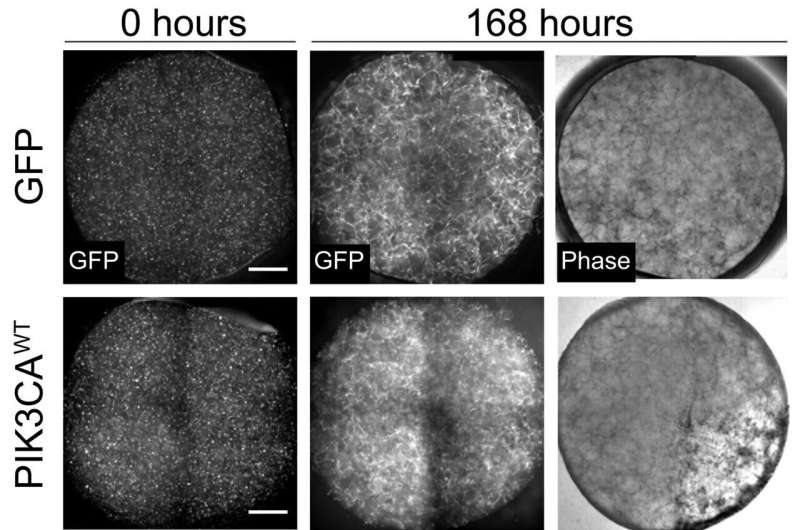
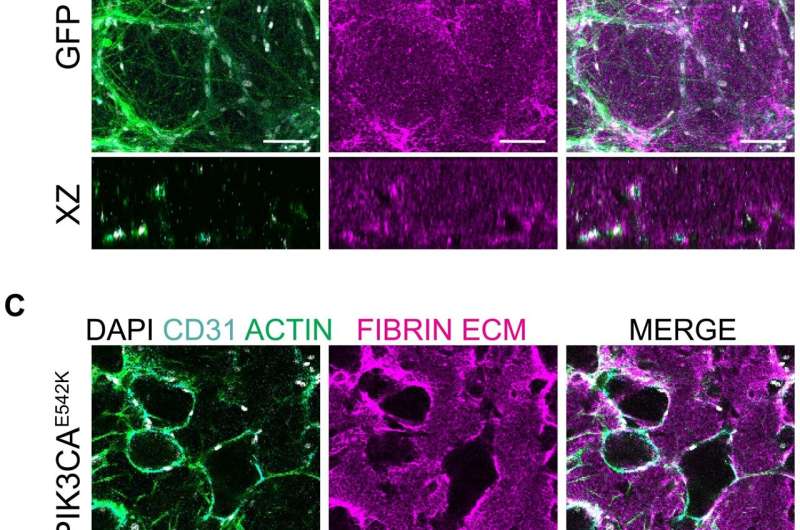
Microfluidic models are incredibly small—about the size of a millimeter—three-dimensional devices that can be used to control, or simulate the environment, within the body. In this case, a small piece of healthy blood vessel tissue is centered inside of the device. From there, the researchers can introduce specific chemicals and mechanical forces to the model to simulate the conditions of the body. They then initiate the PIK3CA mutation.
To confirm whether or not their model accurately portrays the manifestation of the disease, the team had to conduct a drug efficacy study.
There are two drugs that are currently being used for the treatment of vascular malformations: rapamycin and alpelisib. The latter is a newly discovered PIK3CA-specific inhibitor that was recently been approved by the FDA to treat certain types of breast cancer and PIK3CA-related overgrowth spectrum. So far, pre-clinical studies in mouse models and in patients have shown that alpelisib is more effective in reversing vascular malformation defects.
After selecting the two drugs, Polacheck and Awe applied the treatment to their devices. The study was a success.
“The blood vessel used to be really dilated and large,” said Awe, who was first author of the study. “With the treatment, the drug was able to shrink it and, more or less, revert it back to a normal shape and function. We were very excited to be able to replicate some of the results in vitro with the model that we built.”
Moving forward, Awe and Polacheck are looking to replicate the finding in tissues from vascular malformation patients, especially those who don’t have the PIK3CA mutation or don’t have clear genetic information. Their model can now be used to evaluate new medications or to perform synergistic drug studies.
Multiple Paths for Future Study
Now that they know that their model works, Wen and Polacheck plan to use their model to study one facet of the mutation called temporal dynamics, as well as how the mutation affects malformations of the lymphatic tissue.
The disease initially begins with an individual cell that acquires the PIK3CA mutation. Then, much like a chain reaction, the effects of the mutation in that one cell spreads to the surrounding cells until the malformation is fully formed. As their model currently stands, the lab cannot mimic that natural process.
However, Wen is already working on a new and different approach for a microfluidic model. She aims to create a platform that will allow them to start with cells that are healthy, and then “flip on” the mutation, and watch it progress across the tissue of interest. Ultimately, it will help them understand how the mutation is able to affect other cells and move throughout space.
Vascular malformations can also occur in lymphatic tissue. As opposed to blood vessels, lymphatic vessels have a duty to recycle excess fluid throughout the body and acts as a superhighway for immune cells to get to sites of infection. Very little is known about the basic cell biology of lymphatic endothelial cells, so Polacheck is hoping to do a study that is similar to his most recent one.
“The outputs are slightly different because the function of the lymphatics is different from blood vessels,” said Polacheck. “By comparing and contrasting what happens on the blood side and the lymphatic side, we will also be able to learn something about the basic biology of those two types of tissues.”
More information:
Wen Yih Aw et al, Microphysiological model of PIK3CA-driven vascular malformations reveals a role of dysregulated Rac1 and mTORC1/2 in lesion formation, Science Advances (2023). DOI: 10.1126/sciadv.ade8939
Journal information:
Science Advances
Source: Read Full Article


