Gastrointestinal and digestive issues impact roughly 3 million people across the United States alone, and that number is growing. A new study from Scripps Research scientists shows how sensory neurons control our gastrointestinal tracts—critical information that could shape our understanding of related diseases and disorders.
The study, published in the journal Cell on Aug. 3rd, 2023, used a combination of human clinical data and animal models to reveal that the receptor PIEZO2 controls gastrointestinal transit through the stomach, small intestine, and colon by sensing the presence of food and slowing the rate of gut motility accordingly. These findings could lead to therapeutic applications for a range of gastrointestinal conditions, such as Inflammatory Bowel Disease and Irritable Bowel Syndrome.
“PIEZO2 plays a crucial role in gastrointestinal physiology and is necessary for normal gut function,” says senior author Ardem Patapoutian, Ph.D., professor in the Dorris Neuroscience Center at Scripps Research and a Howard Hughes Medical Institute investigator. Patapoutian received the 2021 Nobel Prize in Physiology for discovering that PIEZO2 and a related receptor, PIEZO1, are necessary for the cells to respond to mechanical stimuli. “Food and other ingested contents activate PIEZO2, which in turn dramatically slows gut transit.”
Gut transit time—the rate at which food moves through our gastrointestinal tracts—is essential for digestion, nutrient absorption and waste removal. Optimal digestion requires an optimal transit time: too slow, and you end up with constipation; too fast, and you risk diarrhea. Up until now, there’s been a limited understanding of how sensory pathways guide this process.
Patapoutian’s team decided to investigate whether sensory input from the receptor PIEZO2 plays a role in gut motility. PIEZO2 proteins are activated by mechanical forces or pressure and are found throughout the body, though their role in gastrointestinal motility has not been previously explored. PIEZO2 receptors are also involved in sensing the degree of lung inflation or bladder filling, so it made sense that these receptors might also be able to detect distension of the gastrointestinal tract.
“We wanted to understand the consequences of lacking this mechano-sensation and whether people without PIEZO2 have gastrointestinal problems,” says M. Rocio Servin-Vences, Ph.D., the study’s first author and a postdoctoral fellow in the Patapoutian lab at Scripps Research and the Howard Hughes Medical Institute.
Humans are sometimes—though rarely—born without functional PIEZO2 genes, and studying these individuals provides a window into the protein’s function. In collaboration with Alexander Chesler’s team at the National Institutes of Health (NIH), the researchers assessed the gastrointestinal health and medical history of a cohort of 12 humans, ranging in age from 9 to 42, who carried non-functional variants of the PIEZO2 gene.
Compared to members of the general population, the PIEZO2-deficient individuals reported a range of gastrointestinal dysfunctions including lumpy and watery stools. Notably, six of the individuals reported that they could not sense bowel movements, and five reported using medications to relieve gastrointestinal distress.
“The gastrointestinal dysfunction that these PIEZO2-deficient individuals described was striking,” says Servin-Vences. “It suggests that PIEZO2-deficient individuals have impaired sensation in bowel function that affects their quality of life, and also that the mechanosensitive channel PIEZO2 plays a crucial role in human gastrointestinal physiology and pathophysiology.”
To explore the mechanism behind how PIEZO2 governs gut physiology, the researchers next turned to animal models. They found that when PIEZO2 was removed from sensory neurons, the mice had much faster gut transit times, more frequent defecation and their stools had a higher water content.
“We were able to show that PIEZO2 is important for slowing down gastrointestinal transit,” says Servin-Vences. “When mice do not have PIEZO2 in these neurons, their gastrointestinal transit goes super fast, which has important consequences because it suggests that there is not enough time for proper nutrient absorption.”
Since PIEZO2 is expressed by multiple groups of neurons that innervate the gut, the researchers also wanted to pinpoint which neural pathway is responsible for controlling gut transit time. By using genetic and viral tools to selectively shut down PIEZO2 in two neural pathways—the nodose ganglia and dorsal root ganglia—the researchers were able to show that neurons in the dorsal root ganglia are exclusively responsible for controlling gut motility.
“It was very surprising that neurons from the dorsal root ganglia—but not from the nodose ganglia—have a homeostatic function in gut motility,” says Servin-Vences. “The nodose ganglia is known to regulate many functions in the gut, lungs and heart, whereas neurons from the dorsal root ganglia are more known for innervating the skin and detecting temperature and pain.”
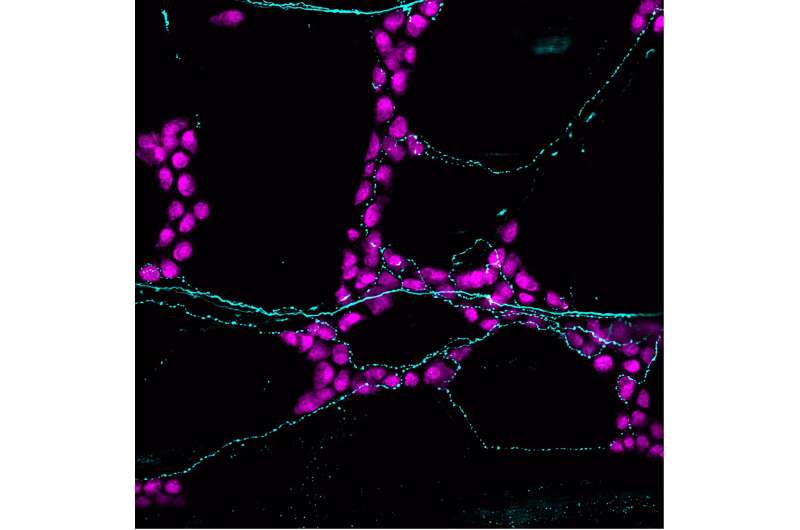
The team also used fluorescent imaging to show that PIEZO2 control of gut motility occurs along the entire gastrointestinal tract—from stomach to small intestine to colon.
Though this is just an initial glimpse into how sensory neurons control gut motility, the researchers say it opens a slew of new research questions, and the possibility for future therapeutics. Servin-Vences says next steps are to assess how diet, lifestyle, stress and even microbiota affect this complicated system.
“In the long term, if we are able to find specific drugs that can either inhibit or stimulate the ion channel, we might be able to tune gut motility, which would have very important implications for gastrointestinal motility disorders,” says Patapoutian.
More information:
Ardem Patapoutian, PIEZO2 in somatosensory neurons controls gastrointestinal transit., Cell (2023). DOI: 10.1016/j.cell.2023.07.006. www.cell.com/cell/fulltext/S0092-8674(23)00739-0
Journal information:
Cell
Source: Read Full Article


