Immune cells that keep the brain free of debris but also contribute to inflammation are the likely culprits behind the concentration and memory problems that sometimes follow one type of chemotherapy, a new study in mice suggests.
Researchers previously showed (in work published in Scientific Reports ) that female mice given paclitaxel, a drug commonly used to treat breast, ovarian and other cancers, developed memory problems that were linked to inflammation in the brain. Mice receiving a placebo did not develop the “mental fog” phenomenon known as chemo brain.
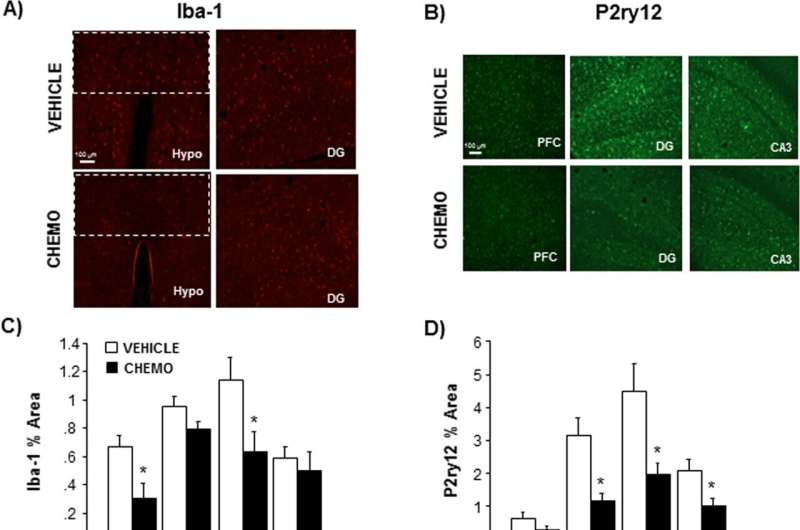
In this study, the team used a technique to delete immune cells called microglia from the brains of mice that had received paclitaxel. The loss of those cells restored the chemo-treated animals’ memory and also lowered inflammation in their brains.
“That was the peak of the paper: We demonstrated that microglia were necessary for the cognitive impairments we’ve seen with paclitaxel—the behavior was reversed,” said senior author Leah Pyter, an investigator in the Institute for Behavioral Medicine Research at The Ohio State University.
“When we got rid of those microglia, it was associated with a reduction in the inflammatory response to chemo,” said Pyter, associate professor of psychiatry and behavioral health in Ohio State’s College of Medicine. “So we think that when microglia are activated and become pro-inflammatory, that’s what is ultimately affecting neurons to impair memory.”
The research was published online recently in the journal Brain, Behavior and Immunity.
In these studies, the mice never have cancer—the research is intended to study only the effects of paclitaxel, which is often combined with one or more additional drugs in regimens determined to provide the most effective treatment for certain types of breast cancer. A member of the Cancer Control Research Program, Pyter is also studying how the gut microbiome may play a role in chemo brain.
Identifying chemotherapy side effects, and the cells and pathways involved, in animal studies is a first step toward proposing potential interventions that could lessen the impact this important cancer treatment has on the body and brain. Chemotherapy agents work by killing cancer cells, but also kill other dividing cells, and the immune system clean-up of resulting debris is thought to drive inflammation, Pyter said.
The team predicts that inflammatory cells in the rest of the body, known as the periphery, are sending signals that activate microglia to become pro-inflammatory in the brain, and those signals interact with cells in the blood-brain barrier—hinting at three potential areas to target.
“We should always be trying to get to more targeted treatments. And the first step of that is to understand who the major players are,” Pyter said. “As we look for potential targets for intervention, we have to keep in mind that cancer patients need their peripheral immune systems intact to respond to tumor cells and get rid of them. So it’s tricky.”
In the new study, mice were given six cycles of paclitaxel injections or a placebo. Researchers found that both microglia and astrocytes, cells in the brain that have an immune role but also perform numerous functions to keep neurons healthy, were activated by the chemotherapy in the hippocampus, the region of focus in this work.
Machine learning analysis showed that compared to mice that received a placebo, microglia in the brains of mice receiving chemo were producing more pro-inflammatory proteins and suppressing a protein important for cognition-related neuron health.
An experimental drug that inhibits a substance that microglia in mice need for survival was added to the animals’ food to deplete microglia in their brains. Chemo-treated mice with normal brain immune cell levels showed memory problems in a standard lab test. In contrast, memory in the mice with depleted microglia was restored, and chemo-induced pro-inflammatory proteins in their brains were significantly reduced.
Drugs similar to this experimental compound have been used in cancer patients receiving paclitaxel to target other types of immune cells, which suggests that temporarily clearing away microglia in humans may be feasible, Pyter said.
“We know that chemotherapy is lifesaving, but comes with the potential for toxicity. A better understanding of how chemotherapy affects the brain opens up research areas and interventions to improve the lives of our cancer patients,” said Dr. Peter Shields, deputy director of The Ohio State University Comprehensive Cancer Center and a practicing thoracic oncologist at The James Cancer Hospital and Solove Research Institute.
Pyter also said the findings hint at possible long-term cognitive effects from paclitaxel because microglia are unusual among immune cell types: They have a long life and don’t repopulate frequently.
“Microglia are always there—they’re very dynamic and they’re trolling for issues. They might look completely normal until they’re activated, and then their response to that activation can be very abnormal,” she said. “We’re using chemo as a hypothesized way to activate them. But say a cancer patient receives and finishes chemo. But later, they have surgery or a huge stressor in their life—that will reactivate those cells and they might respond oddly later in life.”
“This model doesn’t mimic long-term side effects. But it is a big concern because many breast cancer patients survive, and the side effects don’t always go away.”
More information:
Corena V. Grant et al, Microglia are implicated in the development of paclitaxel chemotherapy-associated cognitive impairment in female mice, Brain, Behavior, and Immunity (2022). DOI: 10.1016/j.bbi.2022.12.004
B. R. Loman et al, Chemotherapy-induced neuroinflammation is associated with disrupted colonic and bacterial homeostasis in female mice, Scientific Reports (2019). DOI: 10.1038/s41598-019-52893-0
Journal information:
Brain, Behavior, and Immunity
,
Scientific Reports
Source: Read Full Article


