Recreating native physiological processes in manmade materials imitating the biological structures involved in wound healing has proved to be a lasting challenge. The main problems are modeling the appropriate functions that prompt cell growth, and oversimplified frameworks that do not reflect the complex network of interactions. Researchers from Japan may have found a solution to this puzzle.
In a study published this month in Nature Communications, a research team from Tokyo Medical & Dental University (TMDU) has developed a jigsaw-shaped peptide that performs the basic functions of the extracellular matrix (ECM), serving as an artificial ECM for injured tissue regeneration.
The ECM is a network of biomolecules that facilitates the control and coordination of various cellular events, such as adhesion, migration of signaling molecules, and tissue repair. It achieves this through the binding and release of secreted proteins, including growth factors that stimulate cell growth. Although many artificial ECMs have been reported for tissue regeneration, few studies have explored the development of peptide-based ECM mimics that can both incorporate and release secreted proteins, something the research team at TMDU aimed to address.
“Because of their cell-adhesive properties and ability to degrade into chemically defined molecules, self-assembling hydrogels have great potential for use in clinical applications,” says senior author of the study Itsuki Ajioka. “However, it is difficult to combine the ability to both incorporate and release secreted proteins. This is a challenge that we have worked to overcome in the design of our artificial ECM.”
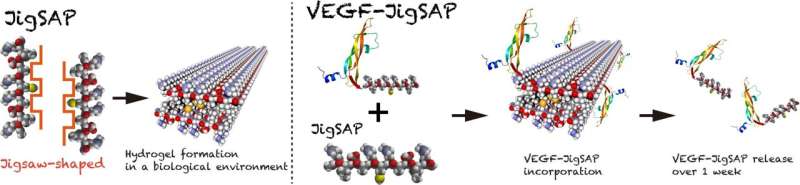
To do this, the researchers designed a jigsaw-shaped self-assembling peptide (JigSAP) that mimics the hydrophobic surface of the dovetail-packing motif of the intracellular protein glycophorin A. JigSAP formed a hydrogel with evenly distributed nanofibers under physiological conditions. The arrangement of these fibers enabled the incorporation and release of vascular endothelial growth factor (VEGF), facilitating regenerative therapeutic effects in a mouse stroke model.
“We rationally designed JigSAP based on structural motifs known to undergo conformational transitions leading to nanofiber formation, and which are found in homodimeric proteins such as glycophorin A,” explains Takahiro Muraoka, senior collaborator from Tokyo University of Agriculture and Technology. “Our characterization of JigSAP in an aqueous environment showed the proper nanofiber distribution in the hydrogel, providing advantageous properties that enabled it to mimic the native ECM functions required for tissue repair.”
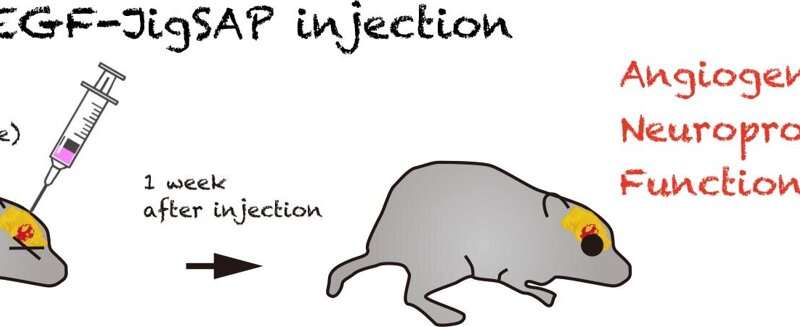
Injection of JigSAP with VEGF—which stimulates the growth of new blood vessels—in a mouse stroke model suggested enhanced blood vessel formation. The mice also demonstrated some functional recovery one week after treatment in a test assessing their post-treatment motor skills.
Source: Read Full Article


