Researchers at the Kobe University Graduate School of Medicine have revealed that alterations in fetal microglia resulting from maternal inflammation could contribute towards the onset of developmental and psychiatric disorders. The research team including Ph.D. student Ozaki Kana and Professor Yamada Hideto from the Department of Obstetrics and Gynecology observed that infant mice that were exposed to maternal immune activation (MIA) displayed changes in microglial process motility during gestation and development. These changes remained after birth and were linked to social behavior deficits, such as those that are found in autism spectrum disorders.
Microglia, the brain’s immune cells, monitor the parenchyma of the brain by extending and retracting their processes. In recent years, controlling the number of neurons and synapses using this movement has received attention. Therefore, process motility is thought to play a vital role in the microglia’s physiological functions. This research sought to investigate how microglial process motility changes in developmental disorders and schizophrenia. The researchers succeeded in illuminating part of the role that these microglial changes play.
The research group compared and analyzed microglial process movements in MIA mice during the gestational, developmental and adolescent stages using two-photon microscopy. They discovered that changes in microglial process motility were present at all stages, revealing that these alterations were related to social behavioral patterns akin to those characteristic of developmental disorders and schizophrenia.
This research was conducted in collaboration with Professor Wake Hiroaki and Associate Professor Kato Daisuke of Nagoya University, and Dr. Andrew J. Moorhouse of The University of New South Wales. The results were published in Scientific Reports on December 7.
Research published in recent years has suggested that maternal inflammation caused by infection and auto-immune diseases during pregnancy increases the risk of developmental disorders or schizophrenia occurring in the offspring. However, few studies on the mechanism by which maternal inflammation increases the risk of these disorders have focused on clarifying the role of the brain’s immune cell microglia in this mechanism.
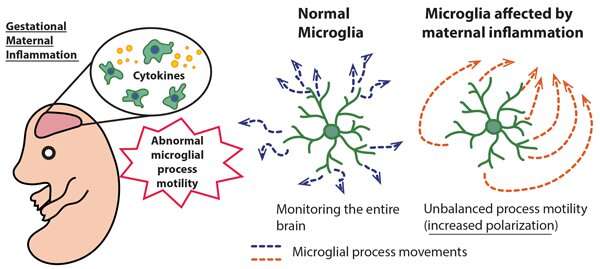
Microglia are the only immune cells in the brain. They originate in the yolk sac and permeate the brain during early embryonic stage. Microglia function as tissue macrophages and distribute themselves inside the brain via repeated division.
Up until now, scientists have focused on finding out how microglia contribute towards the formation of the neural network. In recent years, research has clarified that microglia monitor the brain parenchyma via repeatedly extending and retracting their processes, and this is how they perform their functions on neurons and synapses. As microglia are immune cells, they can also change in response to maternal inflammation.
The current research team revealed that changes to microglial process motility resulting from maternal inflammation during gestation remained after birth. They discovered this by comparing the motility of normal microglia with the microglia from MIA mice. The researchers also illuminated that in adolescence this altered process motility was linked to deficits in social behavior that are characteristic of developmental disorders and schizophrenia.
First, the researchers created MIA mice, which are mice exposed to maternal immune activation in the womb. This was achieved by injecting pregnant mice during the second or third trimesters with a substance to cause inflammation that resembled that which results from a viral infection. They subsequently compared the expression of microglial inflammatory agents (cytokines) during the embryonic and post-natal developmental stages. In both Group 1 (exposed to MIA in the second trimester) and Group 2 (exposed to MIA in the third trimester), there was an increase in microglial inflammatory cytokines in the brains of the fetal mice. In addition, the post-natal development stage changes in the expression of microglia-specific genes were also found in MIA mice.
Observation of microglia in MIA mice under a two-photon microscope showed that there was an increase in microglial process velocity at 18 days gestation for both the second and third trimester groups. However, in both groups microglial process velocity declined at 10 days after birth (i.e. during the developmental stage). Furthermore, the MIA mice that were induced with maternal inflammation during the second trimester demonstrated a tendency towards increasingly polarized microglial process motility. These results suggest the possibility that changes in microglial process motility affect the formation of the neural network during developmental stages.
It is known that inflammation, such as fever, can result in improved social ability in patients with autism. To investigate this, the researchers induced systemic inflammation in normal mice and MIA mice at 42 days after birth (adolescence) and compared the reactions of their microglia to this inflammation. They also evaluated changes in the mice’s social behavior before and after the systemic inflammation was induced. From the results of this experiment, they found that microglial process velocity increased in response to systemic inflammation, and this was the case in both Group 1 and Group 2 MIA mice and in normal mice. However, polarized microglial process motility was seen only in Group 1 MIA mice. Furthermore, this revealed that the increased polarization of process motility is connected to social behavior.
The above findings showed that maternal inflammation affects the fetal microglia during the embryonic stage, resulting in alterations in microglial process motility that begin at the embryonic stage and remain in the developmental stage, or even the adolescent stage. Moreover, these research results demonstrate the possibility of a connection between changes in microglial process motility and deficits in social behavior that are characteristic of developmental disorders and schizophrenia.
This research has revealed that changes in microglial process motility are the key physiological parameter for the effects of maternal inflammation, and that these alterations persist from the embryonic stage up until the developmental or even adolescent stages, which suggests that these alterations affect neuronal network formation. Furthermore, they indicate a possible connection between these changes and social behavioral deficits found in developmental disorders and schizophrenia.
Source: Read Full Article


