The majority of the persisting sequelae in coronavirus disease 2019 (COVID-19) survivors with pulmonary disposition include fatigue, chest pain, and chronic lung disease, all of which directly affect their quality of life.
However, the mechanisms responsible for the development of these late post-acute sequelae of COVID-19 (PASC) are unclear, as the random autopsy samples that are available for examination describe the disease only at single time points. Furthermore, these samples do not provide a longitudinal picture of the processes that could have provided the basis for studies of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pathogenesis.
Considering the urgency to understand the pathogenesis of PASC, researchers have recently developed a mouse model that would provide opportunities to identify early biomarkers and test countermeasures needed to detect and prevent PASC.
Study: A model of persistent post SARS-CoV-2 induced lung disease for target identification and testing of therapeutic strategies. Image Credit: Egoreichenkov Evgenii / Shutterstock.com
Background
COVID-19 is now characterized as a biphasic disease with an acute phase that is characterized by active SARS-CoV-2 infection, followed by a post-viral clearance phase associated with host reparative and protective immunological processes. Approximately 40% of COVID-19 survivors develop PASC; however, the lack of available human longitudinal autopsy samples has limited the understanding of the pathogenesis of PASC-associated lung abnormalities. In such scenarios, animal models provide an excellent platform to overcome these gaps in knowledge.
Mouse models of acute SARS-CoV-2 infection are already available to conduct studies; however, models of PASC-like lung disease phenotypes have not been reported yet. In the current study published on the bioRxiv* preprint server, researchers characterize the spatial and temporal patterns of long-term pulmonary consequences associated with SARS-CoV-2 MA10 (mouse-adapted variant) infection in standard BALB/c laboratory mice.
About the study
The lung disease in mice surviving acute SARS-CoV-2 MA10 infection was monitored for over a period of four months (120 days). PASC outcomes were investigated in 10-week-old mice, as well as the more susceptible mice that were over one year old who were injected intranasally with mouse-adapted SARS-CoV-2 MA10 to induce severe acute disease.
The investigation included complementary virological, histological, and immunological assays on mouse tissue/blood samples, supplemented with immunohistochemistry (IHC) and computed tomography (CT) scanning. To characterize tissue damage and repair, the team utilized digital spatial profiling (DSP) and RNA in situ hybridization (ISH) that examined transcriptional profiles during acute and chronic disease phases. Furthermore, countermeasures for preventing lung disease sequelae of SARS-CoV-2 infection were tested on a newly developed mouse model.
SARS-CoV-2 infected mice displayed acute and chronic pulmonary disease
Pathological investigation revealed that SARS-CoV-2 infection-induced acute lung injury (ALI) in mice, followed by post-acute phase chronic lung sequelae, including chronic active pneumonia (CAP) and pulmonary fibrosis (PF). These findings are similar to those found in the human cases of COVID-19.
Lung fibrotic changes have been observed six months after severe human COVID-19 pneumonia. The SARS-CoV-2 MA10 model replicated this phenotype through 120 days post-infection (dpi).
We developed a model of long-term pulmonary sequelae of SARS-CoV-2 infection that persisted after virus clearance and was more characteristic of the general patient population.”
Both age groups of mice demonstrated 25% mortality; however, young mice cleared the infectious virus earlier than the older mice at seven dpi as compared to 15 dpi, respectively. Similar to humans, surviving mice exhibited subpleural lesions by 30-120 dpi, as characterized by abnormally repairing alveolar type II (AT2) cell presence, immune cell accumulation, abundant myofibroblasts, and collagen deposition, all of which are characteristics of CAP and PF.
Disease severity, however, usually diminished over 120 dpi in younger mice as compared to older mice, in which diseased regions remained constant over the 15 to 120 dpi interval. This finding pointed towards a higher reparation potential in younger mice.
Cytokine analysis of lung tissues and serum samples from both age groups revealed robust cytokine responses to infection. However, as compared to older mice, young mice showed more pronounced acute lung and plasma cytokine responses, including antiviral interferons, thereby suggesting their association with the more rapid viral clearance that was observed in younger mice.
Although most acute cytokine levels returned to normal levels by 30 dpi, focally prolonged upregulation of cytokine signaling, including transforming growth factor β (TGF-β), was observed in sub-pleural fibrotic regions. CT scanning detected heterogeneous subpleural ground-glass opacities along with focal fibrosis in surviving mice. Noticeably, similar cellular and fibrotic features in subpleural regions have also been reported in late stage COVID-19 patients.
Consistent with human data, CD4+/CD8+ lymphocytes increased in SARS-CoV-2-affected areas of mouse lungs, which later developed into peripheral lymphoid aggregations in chronic disease. DSP and flow cytometry data also demonstrated the expansion of the interstitial macrophage population, as seen in humans.
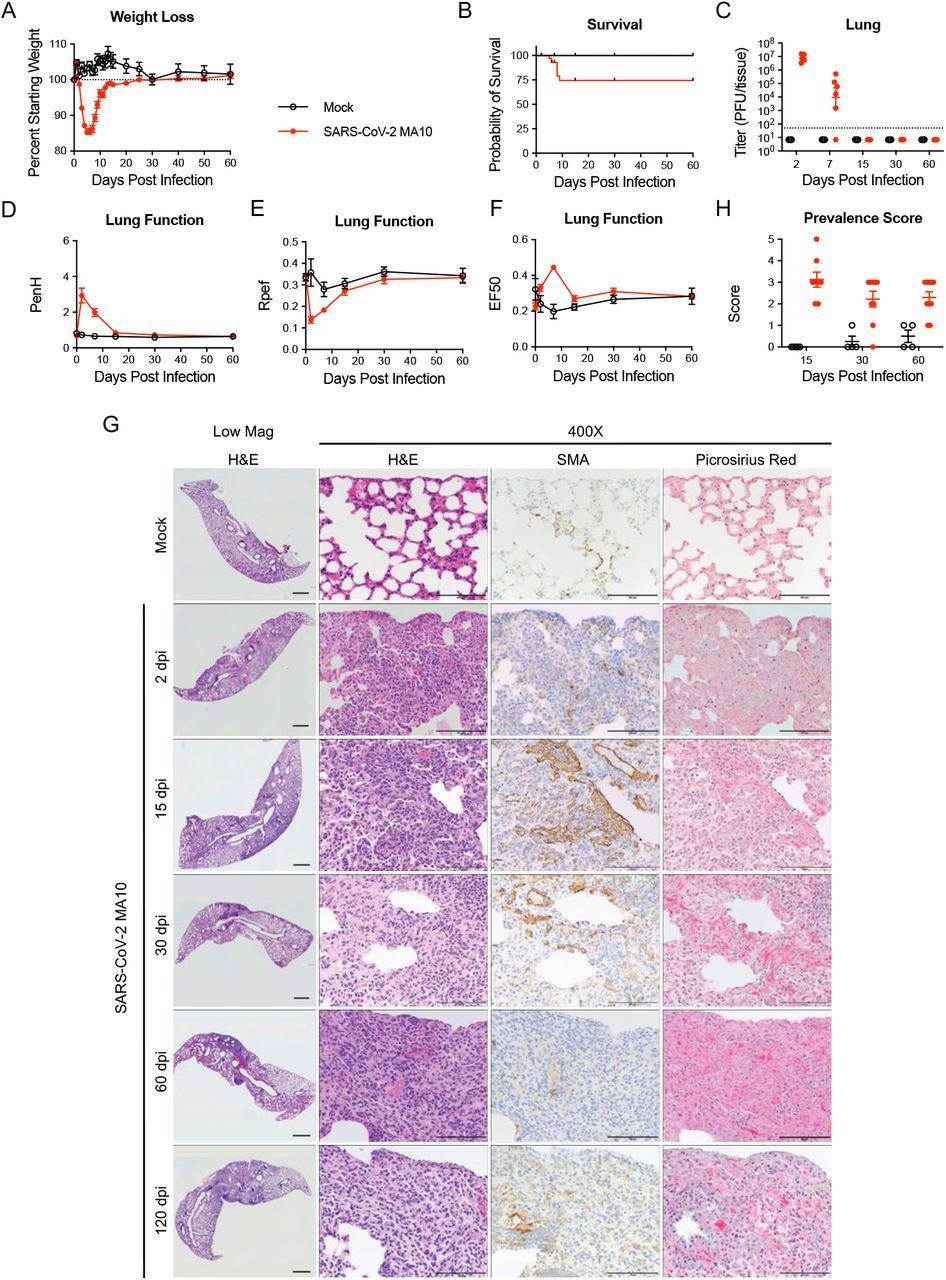
SARS-CoV-2 MA10 infection causes lung damage in aged surviving mice. 1-year-old female BALB/c mice were infected with 103 PFU SARS-CoV-2 MA10 (n=74) or PBS (n=24) and monitored for (A) percent starting weight and (B) survival. (C) Log transformed infectious virus lung titers were assayed at indicated time points. Dotted line represents limit of detection. Undetected samples are plotted at half the limit of detection. (D-F) Lung function was assessed by whole-body plethysmography for (D) PenH, (E) Rpef, and (F) EF50. (G) Histopathological analysis of lungs at indicated time points. H&E: hematoxylin and eosin. SMA: immunohistochemistry for α-smooth muscle actin. Picrosirius Red staining highlights collagen fibers. Image scale bars represent 1000 μm for low magnification and 100 μm for 400X images. (H) Disease incidence scoring at indicated time points: 0 = normal; 0 = 0% of total area of examined section, 1 = < 5%; 2 = 6-10%; 3 = 11-50%; 4 = 51-95%; 5 = > 95%. Graphs represent individuals necropsied at each timepoint (C, H), with the average value for each treatment and error bars representing standard error of the mean (A-H).
The utility of novel mouse model for evaluating antivirals
Animal models of acute and chronic viral diseases are critical for the development of countermeasures. Molnupiravir is a United States Food and Drug Administration (FDA)-approved direct-acting antiviral drug that rapidly clears SARS-CoV-2, while also reducing morbidity, mortality, and time to recovery in humans.
In the current study, the researchers tested Molnupiravir in the SARS-CoV-2 MA10 infected mouse model and observed that an early direct-acting antiviral treatment may prevent chronic lung and other organ PASC manifestations.
The team also tested the early intervention with the anti-fibrotic agent Nintedanib for its ability to reduce the severity of PF following SARS-CoV-2 infection. As compared to controls, Nintedanib administered from 7 dpi reduced fibrotic responses to the virus at 15 dpi, thus supporting the concept that early intervention with anti-fibrotic agents may attenuate the course of PASC.
In summary, the SARS-CoV-2 MA10 mouse model provides novel opportunities to longitudinally study the molecular mechanisms/pathways mediating long-term COVID-19 pulmonary sequelae as relates to human PASC.”
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Dinnon III, K. H., Leist, S. R., Okuda, K., et al. (2022) A model of persistent post SARS-CoV-2 induced lung disease for target identification and testing of therapeutic strategies. bioRxiv. doi:10.1101/2022.02.15.480515. https://www.biorxiv.org/content/10.1101/2022.02.15.480515v1.
Posted in: Medical Science News | Medical Research News | Disease/Infection News
Tags: Antiviral Drug, Blood, CD4, Cell, Chest Pain, Chronic, Chronic Disease, Collagen, Computed Tomography, Coronavirus, Coronavirus Disease COVID-19, covid-19, CT, CT Scanning, Cytokine, Cytometry, Fatigue, Fibrosis, Flow Cytometry, Food, Growth Factor, Hybridization, IHC, Immunohistochemistry, Interferons, Laboratory, Lung Disease, Lungs, Macrophage, Mortality, Mouse Model, Pain, Phenotype, Pneumonia, Pulmonary Fibrosis, Respiratory, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Therapeutics, Tomography, Virus

Written by
Namita Mitra
After earning a bachelor’s degree in Veterinary Sciences and Animal Health (BVSc) in 2013, Namita went on to pursue a Master of Veterinary Microbiology from GADVASU, India. Her Master’s research on the molecular and histopathological diagnosis of avian oncogenic viruses in poultry brought her two national awards. In 2013, she was conferred a doctoral degree in Animal Biotechnology that concluded with her research findings on expression profiling of apoptosis-associated genes in canine mammary tumors. Right after her graduation, Namita worked as Assistant Professor of Animal Biotechnology and taught the courses of Animal Cell Culture, Animal Genetic Engineering, and Molecular Immunology.
Source: Read Full Article