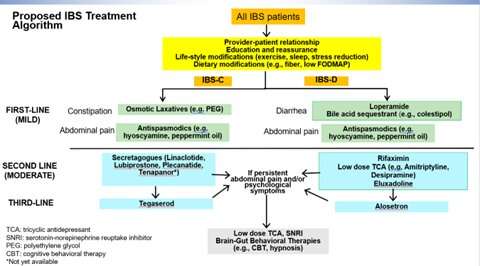
New treatment guidelines released today in Gastroenterology outline a personalized approach for treating patients with approved drug treatments for irritable bowel syndrome (IBS) with constipation (IBS-C) or IBS with diarrhea (IBS-D). IBS is one of the most common disorders of both intestines, affecting up to 35 million Americans.
The guidelines outline, for the first time, when to use newly introduced IBS drugs, when to rely on old drugs approved by the FDA and when to use over-the-counter drugs. With more treatments available, physicians can tailor a personalized approach based on the symptoms a patient with IBS is experiencing.
Source: Read Full Article


