There’s new hope for potentially restoring vision in patients suffering from degenerative retinal disease, thanks to work by researchers at Université de Montréal.
Published this week in Proceedings of the National Academy of Sciences, the research was led by UdeM medical professor Michel Cayouette, director of cellular neurobiology research at the UdeM-affiliated Montreal Clinical Research Institute.
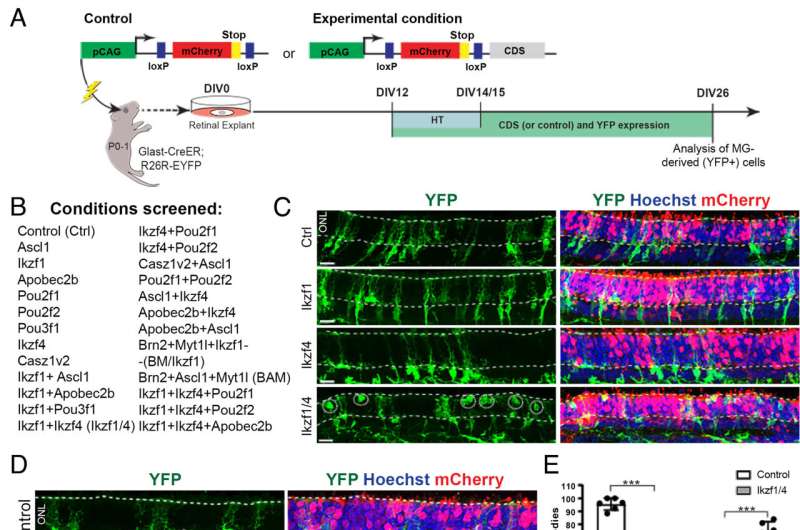
His research team discovered that cells that lie dormant in the retina (glial cells) can be induced to transform into cells sharing some properties with cone photoreceptors, which allow people to do things like perceive colors, read and drive.
Inherited retinal degenerations are caused by the loss of light-sensitive cells in the retina at the back of the eye. When these cells degenerate due to disease, they are not replaced and the patient suffers vision loss that can progress to total blindness.
Although various approaches such as gene therapy exist that offer hope of slowing or blocking the progression of photoreceptor cell loss, these techniques cannot restore lost cells and are therefore not useful for patients at the advanced stages of the disease.
Hence the urgent need to develop regenerative therapies that could replace the lost cells and restore vision. One promising avenue would be to use stem cells to generate photoreceptors that could be transplanted into a patient’s eye, but this technology now faces major challenges that are slowing its being used in clinical practice.
In an approach that circumvents the need for transplantation, Cayouette’s team found a way to reactivate dormant cells in the retina and transform them into neural-like cells that could ultimately be used to replace cells lost in retinal degeneration.
“We have identified two genes that, when expressed in these dormant cells called Müller cells, can convert them into retinal neurons,” said the study’s first author Camille Boudreau-Pinsonneault, who recently earned her Ph.D. at UdeM for this breakthrough.
“What’s interesting is that these Müller cells are known to reactivate and regenerate retina in fish,” she said. “But in mammals, including humans, they don’t normally do so, not after injury or disease. And we don’t yet fully understand why.”
Co-author Ajay David, a doctoral student in Cayouette’s lab, lauded “this exciting advance over cell transplantation,” saying “we may one day be able to take advantage of the cells that are normally present in the retina and stimulate them to regenerate retinal cells lost to pathological conditions and to restore vision.”
Building on their success, the scientists now plan to perfect the effectiveness of this technique and find a way to promote full maturation of cells into cone photoreceptors that could restore vision.
More information:
Camille Boudreau-Pinsonneault et al, Direct neuronal reprogramming by temporal identity factors, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2122168120
Journal information:
Proceedings of the National Academy of Sciences
Source: Read Full Article


