University of Maryland School of Medicine (UMSOM) researchers engineered a new type of CAR T-cell therapy that, in preclinical studies, selectively attacked cancer cells while sparing healthy cells, potentially reducing the likelihood of toxic side effects from this innovative cancer treatment. The cells were designed specifically to attack multiple myeloma, a cancer of the plasma cells found in the body’s bone marrow.
“We could see clearly in our preclinical studies that this new therapy efficiently eliminated the tumor cells without attacking healthy cells. We’re very optimistic about the potential of this as a new and safer cancer treatment,” said study corresponding author Tim Luetkens, MD, Assistant Professor of Microbiology and Immunology at UMSOM. Results were published July 19 in the journal Science Translational Medicine.
About 35,000 Americans are diagnosed every year with multiple myeloma and about 40% of patients die within five years, according to the National Cancer Institute. There are currently two CAR T-cell therapies on the market to treat multiple myeloma that can keep the disease in check for an average of three years. These therapies use the patients’ own genetically engineered immune cells to bind to specific target proteins on the surface of cancer cells. While results from the current CAR T-cell treatments for multiple myeloma are very encouraging, many patients still relapse, demonstrating the need for new treatments.
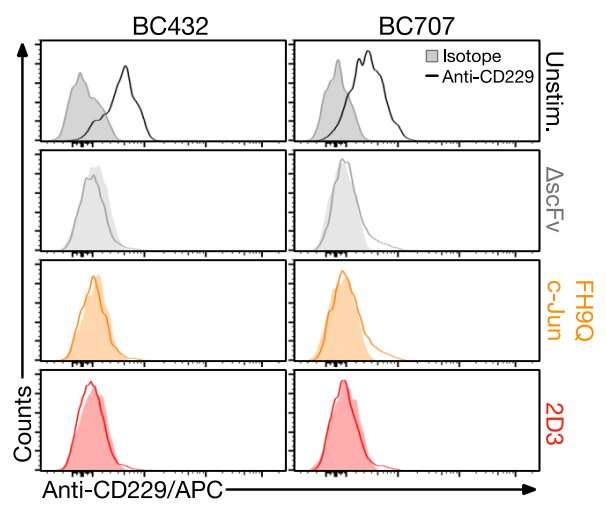
In previous research published in Nature Communications, Dr. Luetkens, Djordje Atanackovic, MD, Professor of Medicine at UMSOM, and Sabarinath Radhakrishnan, MD, Assistant Professor at the Medical College of Wisconsin, developed a new CAR T-cell approach that targets a different protein than current CAR T-cell therapies. They decided to focus on a protein called CD229, which is very prevalent on multiple myeloma cancer cells and less common on healthy cells. However, they demonstrated that CD229 CAR T cells still attacked some healthy blood cells, a potentially serious toxic side effect.
In the current study, Dr. Luetkens and his colleagues aimed to engineer CD229 CAR T cells that only attack the malignant cells but spare healthy cells using a sophisticated approach called affinity tuning. They swapped out single amino acids and tested 305 different variations to painstakingly determine the optimal CAR T-cell sequence that would hit the sweet spot: readily recognizing cancer cells while ignoring healthy cells.
Initially, the researchers were disappointed to see that the optimized receptor or CAR that they had engineered apparently caused the CAR T cells to grow more slowly. This slow growth could, in theory, result in the therapy not being as effective in patients. The research team was able to overcome this hurdle by incorporating a new approach developed by researchers at Stanford University.
“Providing additional copies of a protein naturally present in our CAR T cells, called c-Jun, allowed them to actually grow normally and attack cancer cells over a long period of time,” said study lead author Erica Vander Mause, Ph.D., who was a researcher in Dr. Luetkens lab during the study.
Dr. Luetkens and Dr. Atanackovic are both members of the immunology and immunotherapy research program at the University of Maryland Marlene and Stewart Greenebaum Comprehensive Cancer (UMGCCC), which is a regional and national leader in treating patients with CAR T-cell therapy. The center has treated more than 300 patients with blood cancers, such as large B-cell lymphoma, leukemia and multiple myeloma, since the U.S. Food and Drug Administration approved the first CAR T-cell therapy in 2017.
“Clinical studies are currently being initiated at UMGCCC and partnering institutions to investigate, as a first step, the safety and efficacy of CD229-targeted CAR T cells for the treatment of multiple myeloma and possibly also other malignancies,” said Dr. Atanackovic.
UMGCCC was the first cancer center in Maryland, Virginia and Washington, D.C., to be certified to offer CAR T-cell therapy to patients with aggressive lymphoma. Many of these patients had relapsed and refractory forms of blood cancers, leaving them with no other viable treatment options. More than half of the patients who have received the therapy are alive; some of them for five years and are effectively cured.
UMGCCC also has a robust preclinical and clinical cellular therapy research program, with physician-scientists developing next-generation CAR T-cell therapies for blood cancers and certain solid tumors. As in the latest study, the researchers are also trying to reduce toxic side effects of the treatment and improve its effectiveness. UMGCCC has the distinction of having its own state-of-the art facility GMP-compliant processing facility—the Fannie Angelos Cellular Therapeutics Laboratory, located at UMSOM—capable of producing advanced cell-based therapies and cancer vaccines.
“Optimal CAR binding strength remains an open question in the CAR T cell field, with low-affinity CAR T cells often showing better long-term effectiveness and safety,” UMSOM Dean Mark T. Gladwin, MD, Vice President for Medical Affairs, University of Maryland, Baltimore, and the John Z. and Akiko K. Bowers Distinguished Professor. “This research provides a flexible platform to take existing CARs and optimize their binding strength to potentially make these therapies safer and more effective for different types of cancers.”
More information:
Erica R. Vander Mause et al, Systematic single amino acid affinity tuning of CD229 CAR T cells retains efficacy against multiple myeloma and eliminates on-target off-tumor toxicity, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.add7900
Journal information:
Science Translational Medicine
,
Nature Communications
Source: Read Full Article