A recent study conducted by an international team of scientists has revealed that the Oxford-AstraZeneca coronavirus disease 2019 (COVID-19) vaccine, AZD1222, is capable of inducing strong spike-specific CD4+ T cell helper type 1 (Th1) and CD8+ T-cell responses in vaccinated individuals. A detailed description of AZD1222-induced cellular immunity is currently available on the medRxiv* preprint server.
Background
The Oxford-AstraZeneca-developed AZD1222 is a replication-deficient adenovirus vector-based COVID-19 vaccine, which has shown significant efficacy in preventing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and symptomatic COVID-19 in both clinical trials and real-world pandemic situation.
Besides inducing robust anti-spike antibody responses, AZD1222 has been found to trigger strong spike-specific effector T cell responses in adults at day 14 post-vaccination.
Studies characterizing AZD1222 efficacy have revealed that T cell responses can be detected as early as day eight and maintained through day 56 post-vaccination. However, AZD1222 has not yet been characterized extensively in terms of T cell cytokine responses.
In the current study, the scientists have characterized functional CD4+ and CD8+ T-cell responses in healthy adults after administering 1st and 2nd doses of the AZD1222 vaccine. Moreover, they have described the breadth and depth of spike-specific T cell responses induced by AZD1222.
A total of 280 participants who received two doses of AZD1222 vaccine were analyzed for spike-specific cytokine secretion and T cell receptor sequencing.
Important observations
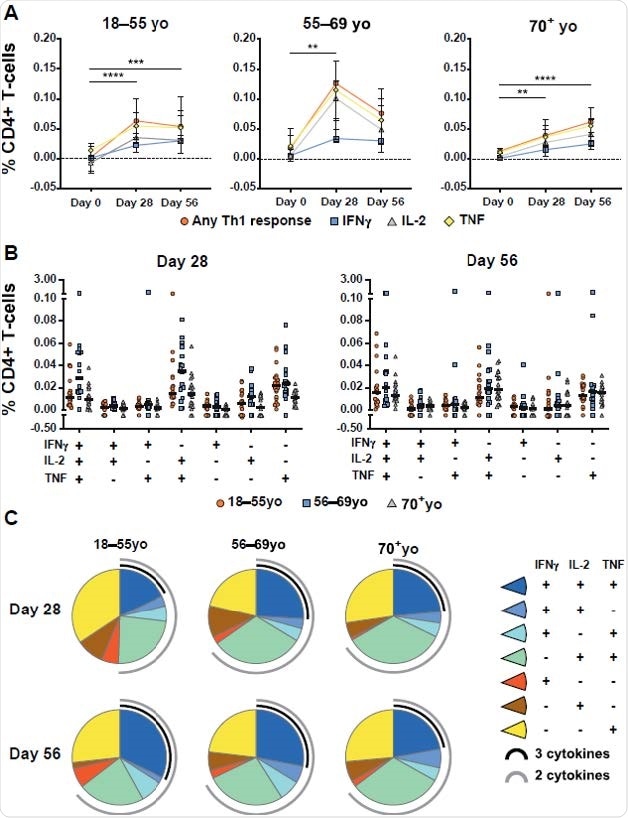
To determine spike-specific T cell response in vaccinated individuals, the scientists experimentally stimulated cytokine production in participant-derived peripheral blood mononuclear cells with peptide pools that cover the entire spike sequence of SARS-CoV-2. The findings revealed that compared to the baseline (day 0), the total spike-specific CD4+ T cell helper type 1 (Th1) response increased significantly at days 28 and 56 post-vaccination.
The cytokines produced by Th1 cells primarily included TNF, followed by Interleukin-2 (IL-2), and Interferon-gamma (IFNγ). However, no Th2 response was observed in AZD1222-vaccinated individuals.
In contrast to CD4+ Th1 responses, a significantly lower frequency of spike-specific CD8+ T cells was detected in vaccinated individuals at days 28 and 56 post-vaccination.
A significant difference in cytokine production pattern was also observed between CD4+ and CD8+ T cells, with CD8+ T cells primarily producing IFNγ, followed by TNF and IL-2.
Together, these observations suggest that AZD1222 induces robust Th1 responses and significant expansion of CD8+ T cell responses.
Although a significantly increased CD4+ T cell response was observed across all age groups tested (18–55, 56–69, and ≥70 years), the kinetics of Th1 response was found to vary with age. Specifically, the findings revealed that AZD1222 induces polyfunctional Th1 response and CD8+ T cell response with similar frequency and functionality at day 56 post-vaccination across all age groups tested.
To determine the diversity and specificity of spike-specific T cell responses, the scientists performed sequencing of T cell receptor beta chain on peripheral blood mononuclear cells derived from AZD1222-vaccinated individuals at day 0 and day 28 post second dose. The findings revealed that after receiving the 2nd vaccine dose at 4 weeks or 12 weeks interval, the participants had significantly increased fractions of spike-specific total T cell receptors (depth of the response) and unique T cell receptors (breadth of the response).
Specifically, a significantly increased spike-specific T cell receptor breadth was observed at day 28 post second dose across all age groups tested. Similarly, a significantly increased spike-specific T cell receptor depth was observed at day 28 post second dose.
The mapping of each unique spike-specific T cell receptor sequence to a specific spike protein region revealed that spike-specific CD4+ T cell responses spanned the entire spike protein, with dominant responses observed in the N-terminal region 160–218 the C-terminal region 743–854.
In summary, these results suggest AZD1222 vaccination results in a significant expansion of spike-specific T cells whose receptor sequences map to multiple epitopes in the spike protein.
Study significance
The study reveals that the AZD1222 vaccine could provide long-lasting protection against COVID-19 by significantly inducing polyfunctional Th1-dominated T cell responses to SARS-CoV-2 spike protein. Moreover, the vaccine causes a significant expansion of spike-specific CD4+ and CD8+ T cells with unique T cell receptor sequences that map to multiple spike epitopes.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Swanson II PA. 2021. T-cell mediated immunity after AZD1222 vaccination: A polyfunctional spike-specific Th1 response with a diverse TCR repertoire. medRxiv. https://doi.org/10.1101/2021.06.17.21259027, https://www.medrxiv.org/content/10.1101/2021.06.17.21259027v1
Posted in: Medical Research News | Disease/Infection News
Tags: Adenovirus, Antibody, Antigen, Blood, CD4, Cell, Coronavirus, Coronavirus Disease COVID-19, Cytokine, Cytokines, Efficacy, Frequency, Interleukin-2, Pandemic, Protein, Receptor, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, T-Cell, Vaccine

Written by
Dr. Sanchari Sinha Dutta
Dr. Sanchari Sinha Dutta is a science communicator who believes in spreading the power of science in every corner of the world. She has a Bachelor of Science (B.Sc.) degree and a Master's of Science (M.Sc.) in biology and human physiology. Following her Master's degree, Sanchari went on to study a Ph.D. in human physiology. She has authored more than 10 original research articles, all of which have been published in world renowned international journals.
Source: Read Full Article