Skeletal muscle plays an extremely important role in supporting movement and energy metabolism. However, its function can be impaired by aging and muscle-related diseases, resulting in decreased endurance or strength of skeletal muscle.
Skeletal muscle function decreases with the increase of age or the development of disease. After the age of 30, muscle mass decreases by about 3%–8% every decade. Duchenne muscular dystrophy (DMD), a common muscular dystrophy in children, causes proximal muscle weakness and calf hypertrophy, eventually leading to degeneration of skeletal and cardiac muscle. Therefore, healthy skeletal muscle is very important to the body. However, skeletal muscle is still one of the least adequately treated organs with drugs.
Recently, Prof. Chen Chang from Institute of Biophysics of the Chinese Academy of Sciences (CAS), Prof. Ye Yang and Prof. Chen Kaixian’s group from Shanghai Institute of Materia Medica of CAS, revealed the new function of naringenin (NAR) to enhance muscle endurance and improve muscle atrophy by activating Sp1-ERRγ transcriptional axis. The study was published online in Cell Reports.
Previous studies by Prof. Chen’s group showed that Lycium barbarum could improve muscle endurance by increasing oxidative myofiber content and aerobic metabolism, and NAR played a significant role in determining its active components. NAR is a natural plant-derived dihydroflavonoid that has shown effects in metabolic syndrome, inflammation, cancer, and nervous system diseases. However, the direct target of NAR is still unknown, which greatly limits the functional research and application of NAR.
Using 2-month-old young mice, 10-month-old middle-aged mice and mdx mice (DMD disease mice) as models, the researchers found that NAR improved muscle endurance by increasing oxidative myofiber content and aerobic metabolism. During the critical period when the muscle function of middle-aged mice begins to decline, NAR can simultaneously increase skeletal muscle mass and endurance. In the mdx muscle disease model mice, NAR significantly alleviated muscle damage both morphologically and functionally, suggesting its crucial role in alleviating age-related or disease-associated muscle atrophy.
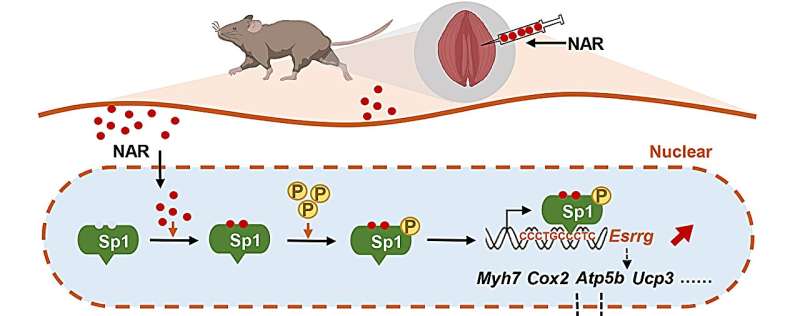
How does NAR play a role in skeletal muscle? Through affinity pull-down assays using biotin-labeled naringenin (NAR-biotin), mass spectrometry identification and drug design analysis, the researchers found that the GLN-110 site of transcription factor Sp1 can interact with NAR via hydrogen bond, and Sp1-AAV and Sp1 inhibitor Mit-A could block the endurance-enhancing effect of NAR in mice. They also found that NAR could significantly up-regulate the expression of transcription factor Esrrg in the above three animal models and cells, and the inhibition of Esrrg could block the effect of NAR on oxidative myofiber content.
To further determine the molecular mechanism behind the binding of NAR to Sp1 and regulating the expression of Esrrg, the researchers carried out mutation assays, truncation experiments, inhibition of binding sites, and chromatin immunoprecipitation. They demonstrated that NAR elevates the phosphorylation level of Sp1, enhancing its binding to the Esrrg promoter sequence, thereby upregulating Esrrg expression to bolster muscle function.
This study found that NAR has a new function in improving skeletal muscle quality and endurance. It provides new ideas on alleviating muscle atrophy caused by DMD, lays a theoretical foundation for the application of NAR, and provides a new idea for the drug screening for improving skeletal muscle dysfunction.
More information:
Zhenyu Lv et al, Naringenin improves muscle endurance via activation of the Sp1-ERRγ transcriptional axis, Cell Reports (2023). DOI: 10.1016/j.celrep.2023.113288
Journal information:
Cell Reports
Source: Read Full Article


