Neuropathic pain is a debilitating condition estimated to affect as much as 10% of the global population. It results from a damaged or malfunctioning nervous system, stemming from diseases like diabetes, alcoholism, strokes, Parkinson’s and other causes like spinal nerve compression, radiation and chemotherapy treatments.
Management of neuropathic pain has been particularly challenging as currently prescribed drugs, such as anticonvulsants and antidepressants can have severe adverse effects, are not well-tolerated, take a longer time to work only help a subset of patients. Opioids come with the risk of physical dependence and addiction.
Researchers from Boston University Chobanian & Avedisian School of Medicine in collaboration with the Icahn School of Medicine, Columbia University and the New York State Psychiatric Institute used experimental models to explore the cellular actions of tianeptine and provide insight for the development of more effective and safer treatments of neuropathic pain conditions.
In a study published today in the journal Neuropsychopharmacology the investigators report that the atypical antidepressant tianeptine potentially provides rapid and lasting pain relief with a low risk of addiction.
“We hope this revives the potential of using tianeptine for the treatment of chronic pain and associated conditions, such as anxiety and depression,” said Venetia Zachariou, Ph.D., corresponding author of the study and professor and Edward Avedisian Chair of Pharmacology, Physiology & Biophysics at Boston University Chobanian & Avedisian School of Medicine. “By further refining this molecule, we could arrive at a pain treatment that is more effective, fast acting, and has a mild side effect profile.”
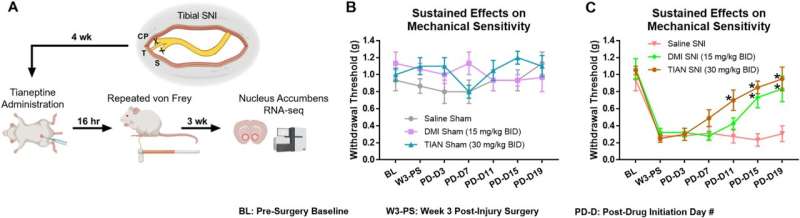
Researchers used experimental models to investigate the persistent mechanical allodynia that is associated with damaged sciatic nerve, comparing the therapeutic profiles of tianeptine to that of the antidepressant desipramine.
To better understand the mechanisms underlying the drug’s effects, the investigators used RNA sequencing, to monitor gene expression changes in the nucleus accumbens, a brain region involved in motivation, addiction, and pain perception to identify pain-related genes that tianeptine treatment counteracts.
The results showed that tianeptine had profound pain-relieving properties that lasted well after the drug was no longer present, which suggested it was the drug affected the expression of genes that were critical for the maintenance of pain symptoms. The researchers also found that tianeptine started working more quickly than other antidepressants.
Tianeptine is prescribed in some European, Asian and Latin American countries in low doses to treat depression, and in higher doses for asthma, anxiety and other conditions. Since tianeptine actions require endogenous opioid receptors; U.S. regulators have not approved it for use here.
“Several studies have shown that the abuse potential of tianeptine is substantially lower than other currently abused opioids such as oxycodone. Furthermore, also based on prior studies, tianeptine does not appear to cause tolerance, so the dose does not need to be increased drastically over time as we see with opioids, which contributes to physical dependance and addiction,” said Zachariou and Alex Serafini, Ph.D., a postdoc in BU’s pharmacology, physiology & biophysics department and first author of the paper.
“The data from this study contributes to a much larger set of data supporting the use of this drug for conditions such as chronic pain.”
More information:
Randal A. Serafini et al, Tianeptine promotes lasting antiallodynic effects in a mouse model of neuropathic pain, Neuropsychopharmacology (2023). DOI: 10.1038/s41386-023-01645-w
Journal information:
Neuropsychopharmacology
Source: Read Full Article