An old antibiotic may provide much-needed protection against multi-drug resistant bacterial infections, according to a new study published May 16th in the open access journal PLOS Biology by James Kirby of Harvard Medical School, US, and colleagues. The finding may offer a new way to fight difficult-to-treat and potentially lethal infections.
 Study: Streptothricin F is a bactericidal antibiotic effective against highly drug-resistant gram-negative bacteria that interacts with the 30S subunit of the 70S ribosome. Image Credit: Kateryna Kon / Shutterstock
Study: Streptothricin F is a bactericidal antibiotic effective against highly drug-resistant gram-negative bacteria that interacts with the 30S subunit of the 70S ribosome. Image Credit: Kateryna Kon / Shutterstock
Nourseothricin is a natural product made by a soil fungus, which contains multiple forms of a complex molecule called streptothricin. Its discovery in the 1940s generated high hopes for it as a powerful agent against Gram-negative bacteria, which, due to their thick outer protective layer, are especially hard to kill with other antibiotics. But nourseothricin proved toxic to kidneys, and its development was dropped. However, the rise of antibiotic-resistant bacterial infections has spurred the search for new antibiotics, leading Kirby and colleagues to take another look at nourseothricin.
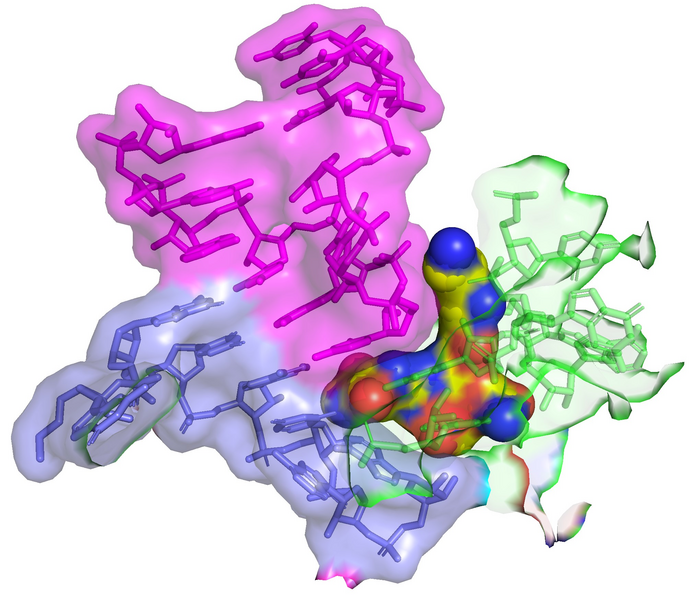
Streptothricin-F (yellow spheres) bound to the 16S rRNA (green) of the bacterial ribosome impinges on the decoding site where tRNA (purple) binds to the codon of the mRNA (blue). This interaction leads to translation infidelity (scrambled protein sequences) and the resulting death of the bacterial cell. The image was created by overlay of PDB 7UVX containing streptothricin-F (this manuscript) with PDB 7K00 containing mRNA and A-site tRNA (ref DOI: 10.7554/eLife.60482). Image Credit: James Kirby (CC-BY 4.0, https://creativecommons.org/licenses/by/4.0/); Zoe L Watson et al., 2023, eLife, CC-BY 4.0 (https://creativecommons.org/licenses/by/4.0/)
Early studies of nourseothricin suffered from incomplete purification of the streptothricins. More recent work has shown that the multiple forms have different toxicities, with one, streptothricin-F, significantly less toxic while remaining highly active against contemporary multidrug-resistant pathogens. Here, the authors characterized the antibacterial action, renal toxicity, and mechanism of action of highly purified forms of two different streptothricins, D and F. The D form was more potent than the F form against drug-resistant Enterobacterales and other bacterial species but caused renal toxicity at a lower dose. Both were highly selective for Gram-negative bacteria.
Using cryo-electron microscopy, the authors showed that streptothricin-F bound extensively to a subunit of the bacterial ribosome, accounting for the translation errors these antibiotics are known to induce in their target bacteria. Interestingly, the binding interaction is distinct from other known inhibitors of translation, suggesting it may find use when those agents are not effective.
"Based on unique, promising activity," Kirby said, "we believe the streptothricin scaffold deserves further pre-clinical exploration as a potential therapeutic for the treatment of multidrug-resistant, Gram-negative pathogens."
Kirby adds, "Isolated in 1942, streptothricin was the first antibiotic discovered with potent gram-negative activity. We find that not only is it activity potent, but that it is highly active the hardiest contemporary multidrug-resistant pathogens and works by a unique mechanism to inhibition protein synthesis."
- PLoS Biology
- Streptothricin F is a bactericidal antibiotic effective against highly drug-resistant gram-negative bacteria that interacts with the 30S subunit of the 70S ribosome Morgan CE, Kang YS, Green AB, Smith KP, Dowgiallo MG, et al. (2023) Streptothricin F is a bactericidal antibiotic effective against highly drug-resistant gram-negative bacteria that interacts with the 30S subunit of the 70S ribosome. PLOS Biology 21(5): e3002091. https://doi.org/10.1371/journal.pbio.3002091, https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3002091
Posted in: Medical Science News | Life Sciences News | Medical Research News | Disease/Infection News
Tags: Antibiotic, Bacteria, Cell, Codon, Electron, Electron Microscopy, Medical School, Microscopy, Molecule, Protein, Protein Synthesis, Research, Ribosome, Translation
Source: Read Full Article