Adding a molecular anchor to the key protein used to recognize cancer in cellular immunotherapies can make the treatments significantly more effective. Scientists at St. Jude Children’s Research Hospital found that immune cells with the anchored protein increased cancer killing, regardless of their cell type or the kind of cancer targeted.
The molecular anchor concept is a new design for improving chimeric antigen receptor (CAR)-based-immunotherapies. CARs have shown some promise in the clinic, but have yet to deliver widespread success across tumor types. The findings were published today in Nature Biotechnology.
“We’ve come up with a new way to more efficiently and effectively bind and target cancer cells,” said first and corresponding author Peter Chockley, Ph.D., St. Jude Department of Bone Marrow Transplantation and Cellular Therapy. “The anchor domain design is modular, universal and cross-species. We showed it worked in multiple CARs and multiple immune cell types—including both Natural Killer (NK) and T cells.”
Scientists can reprogram human immune cells to target cancer cells by adding engineered CAR proteins to their surface. CAR T cells have shown some success in the clinic treating certain cancers, such as relapsed leukemia. However, CAR T cells have failed to deliver for solid tumors, due partially to problems with immune cell activation.
The St. Jude group found a way to “anchor” the CAR molecule within immune cells, allowing the cells to become activated more easily and kill cancer more effectively than conventional CARs. The anchored CARs increased survival in animal models of multiple tumor types, including lung, bone and brain cancers.
“The anchor domain discovery is easily translatable into early phase clinical testing,” said senior author Stephen Gottschalk, M.D., St. Jude Department of Bone Marrow Transplantation and Cellular Therapy chair. “It doesn’t require any other new technology. We strongly believe that this approach needs to get tested in the clinic because no one has tried it before, and it looks very promising in our preclinical work.”
Carefully structured CARs kill cancers better
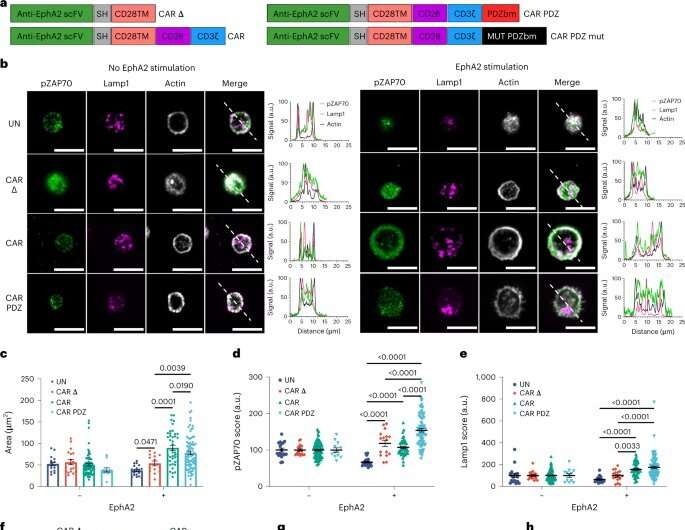
CARs are the key molecule to the cancer-killing process. The outside of the molecule recognizes a protein on the cancer cell. This forms a complex of molecules and proteins between the two cells called the immune synapse. Once the immune synapse is formed, the part of the CAR inside of the immune cell receives signals from the portions outside the cell. These interactions send the ‘go’ signal to activate and kill the cancer cell, however these complex communications can be difficult for conventional CAR T and NK cells to interpret.
“Our approach is different because it focuses on organization,” Chockley said. “CAR structure, when it forms the immune synapse, is very disorganized. The anchoring domain we added organizes the internal scaffolding and makes a better signal, and then brings in other more natural adaptor signaling proteins. The simple addition of organization improves CARs dramatically.”
Picture the back for a desktop computer, with many tangled cords and cables. CAR activation requires untangling the cords and cables to add a monitor. If the cords are disorganized, it can be incredibly difficult. If someone has organized the cables well a task that may have taken hours becomes trivially easy.
Conventional CARs develop a disorganized immune synapse like a desk covered in a mess of cords, causing unclear signaling within the cells. By adding an anchoring domain to the bottom side of the CAR, the scientists organized the CARs—the molecular equivalent of adding cable ties to organize computer cables.
An attractive approach to improve any CAR system
“The most attractive thing about this approach,” said senior author Stephen Gottschalk, M.D., St. Jude Department of Bone Marrow Transplantation and Cellular Therapy chair, “Is that you can put it into any CAR you like. The engineering is simple and easily translatable into lots of different systems.”
The scientists added the molecular anchor by including just four extra amino acids to the end of the conventional CAR. These amino acids then bound the protein Scribble, which is involved in signaling and attaching to the internal structure of the cell. The proteins, amino acids and signaling involved would be familiar to those working in skin (epithelial) cells, but not necessarily to an immunologist working on CARs.
The discovery required technical expertise across at least three different major disciplines, which Chockley has. His work shows that innovation in the field of CAR cells can significantly benefit from paying attention to other fields to find new ways to order and tune the immune synapse.
“There’s a lot more to an immune cell-cancer cell interaction than we’ve been working with,” Chockley said. “We’re entering a new design realm with this domain. We have plenty to do now. There’s a lot to explore.”
More information:
Peter Chockley, Synapse-tuned CARs enhance immune cell anti-tumor activity, Nature Biotechnology (2023). DOI: 10.1038/s41587-022-01650-2. www.nature.com/articles/s41587-022-01650-2
Journal information:
Nature Biotechnology
Source: Read Full Article