The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) triggered the coronavirus disease 2019 (COVID-19) pandemic, which has claimed over 5.8 million lives as of February 14, 2022.
SARS-CoV-2 binds to human cells through the use of its spike protein, which attaches to the human angiotensin-converting enzyme 2 (ACE2) receptor present on the surface of host cells. In a recent Proceedings of the National Academy of Sciences of the United States of America study, researchers suggest that extracellular vimentin (VIM) may act as a coreceptor to allow for SARS-CoV-2 entry into cells.
Study: Extracellular Vimentin Is an Attachment Factor That Facilitates SARS-Cov-2 Entry into Human Endothelial Cells. Image Credit: Kateryna Kon / Shutterstock.com
Introduction
VIM is a filamentous protein found in mesenchymal derivatives, including endothelial cells. To this end, this protein is present at the extracellular surface of endothelial cells and macrophages.
Earlier studies have demonstrated that VIM is an attachment factor for SARS-CoV, Japanese encephalitis virus, and dengue virus. Thus, these observations could indicate the key role of VIM in promoting the entry of SARS-CoV-2 into human circulation and other organs.
SARS-CoV-2 has been identified in the lungs, cardiovascular system, brain, heart, liver, and kidneys of infected humans. Although the ACE2 receptor is abundantly present in the upper respiratory tract, its concentration in the lower respiratory tract is low. Comparatively, many other potential receptors of SARS-CoV-2 have been identified, including Neuropilin-1, CD209L/L-SIGN, CD209/DC-SIGN, and heparan sulfate, which may facilitate its entry into lung cells.
Study findings
The current study confirmed that the SARS-CoV-2 spike receptor-binding domain (RBD) binds to VIM, which is present as intermediate filaments on the outer surface of cells and in the cytoplasm. However, cells expressing VIM did not show increased viral entry as compared to those expressing ACE2.
However, when both VIM and ACE2 were expressed together, viral entry into the cells was significantly increased as compared to ACE2 only-expressing cells. The same result was observed with the addition of exogenous VIM to ACE2-expressing cells. Further research showed that extracellular VIM is a potential coreceptor or attachment factor for SARS-CoV-2.
The researchers then determined whether VIM was present at the same location as ACE2 on cells co-expressing both of these receptors. To this end, they found VIM filaments in the cell membrane, especially where cells were in contact.
Moreover, RBD bound more strongly to cells expressing both molecules, even when recombinant soluble ACE2 was used to test RBD binding with or without VIM. The remarkable increase in RBD-ACE2 binding in the presence of VIM may be due to a different binding site for VIM on the RBD than for ACE2.
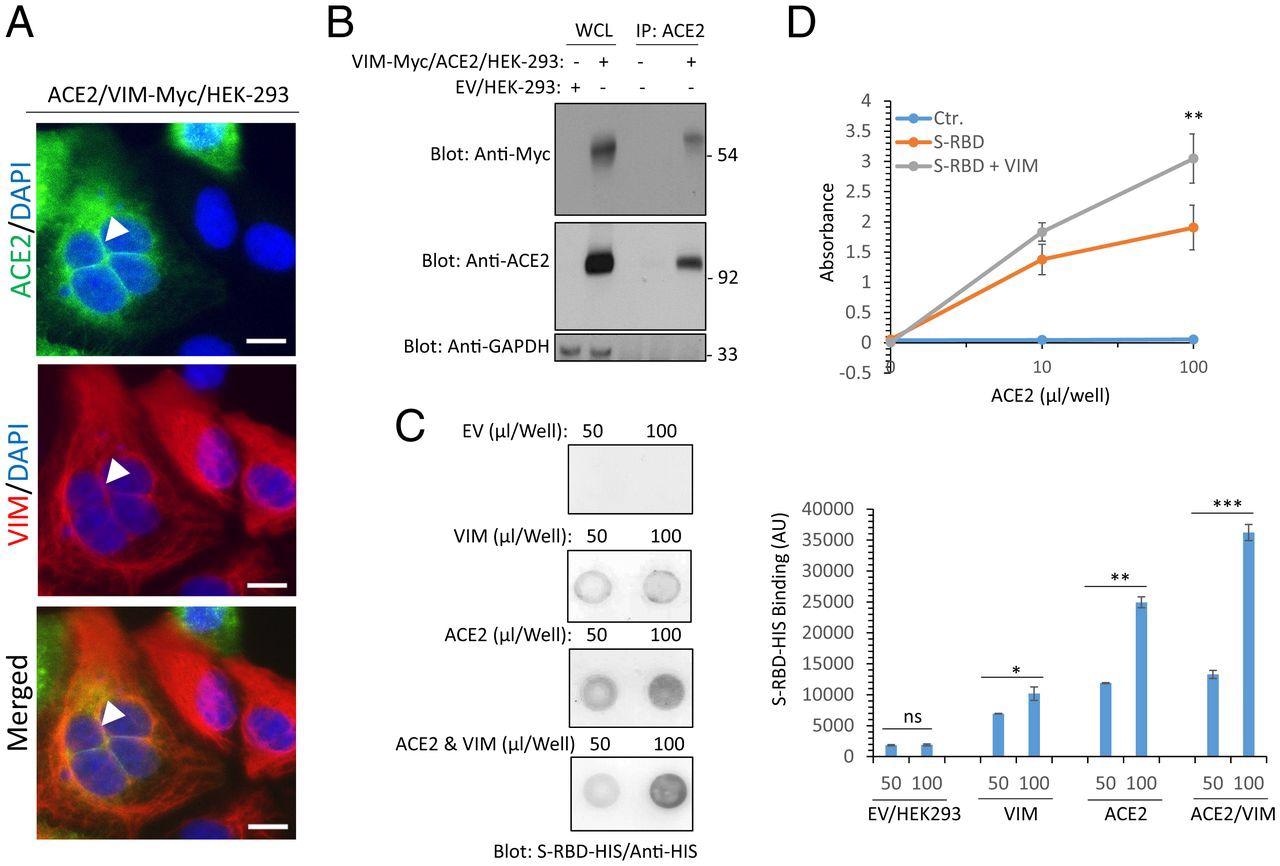
Extracellular VIM facilitates SARS-CoV-2 entry into human cells. (A) HEK-293 cells expressing ACE2 and VIM-Myc were costained with anti-ACE2, anti-VIM and DAPI. The slides were viewed by confocal microscopy. White arrowheads show colocalization of VIM with ACE2. (Scale bars, 50 µM.) (B) WCL from HEK-293 cells expressing control EV or coexpressing VIM-Myc with ACE2 were subjected to a coimmunoprecipitation assay using anti-ACE2 antibody. The immunoprecipitated proteins were analyzed by Western blot analysis using anti-Myc antibody for VIM. The same membrane was also probed for ACE2 and GAPDH. (C) Different concentrations of WCL from HEK-293 cells expressing EV, VIM, ACE2, or coexpressing VIM with ACE2 were blotted on the PVDF membrane. The membranes after blocking with BSA were incubated with S-RBD-HIS-STRP (1 µg/mL), followed with immunoblotting with anti-HIS antibody. Quantification of the blots is shown. (D) A 96-well plate coated with soluble ACE2 was incubated with RBD-HIS-STRP alone or RBD-HIS-STRP with CM containing VIM. The plate was subjected to ELISA and the binding of S-RBD with ACE2 determined with streptavidin-HRP. *P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant.
The researchers also tested the competitive binding of the RBD with ACE2 in the presence of two anti-RBD antibodies CR3022 and S309. Both CR3022 and S309 were found to bind outside the receptor-binding motif (RBM), without directly clashing with ACE2 for binding.
If the RBD is first treated with CR3022, VIM-RBD binding is reduced; however, this same effect was not observed with S309. This may indicate that VIM and ACE2 bind to the RBD at two different sites. Furthermore, CR3022 may depend on the presence of VIM for its neutralizing activity, as incubation of the RBD with this antibody prevents viral entry into VIM+ cells, but not when VIM is knocked out.
“Taken together, our data demonstrate the CR3022 antibody interferes with the binding of SARS-CoV-2-S and can function as a neutralizing antibody in relevant cell types when both VIM and ACE2 are expressed.”
SARS-CoV-2 infection occurred at a higher rate in cells expressing both ACE2 and VIM than ACE2 alone, while VIM knockdown reduced infection rates. This indicates the requirement for VIM for endothelial cell infection by SARS-CoV-2, in which case it could be targeted to prevent or treat SARS-CoV-2 infection. Moreover, VIM may be the bridge binding the spike protein to the ACE2 receptor to promote viral entry into the cell.
When the level of VIM expression was increased, the rate of infection was moderately increased. Comparatively, a significant increase was observed when ACE2 and VIM were both present.
Implications
The results show the key function of VIM as an attachment and infection factor for SARS-CoV-2 into ACE2-expressing cells. In addition to lung cells, SARS-CoV-2 attacks endothelial cells and can also cause damage to the cardiovascular system, kidneys, and intestine.
The endothelial component of infection by SARS-CoV-2 may be an important part of the pathologic process in COVID-19 since endothelial activation can combine with the inflammatory response in COVID-19. This causes a hyper-inflammatory and procoagulant state that results in thrombosis, capillary occlusion within the lungs, abnormal blood vessel growth, as well as neurological features.
Another recent preprint also suggested that VIM is instrumental in SARS-CoV-2 infection since anti-VIM antibody administration prevents infection of ACE2-expressing cells by SARS-CoV-2. With the same binding sites for both, a higher level of expression of VIM would have reduced RBD-ACE2 binding by competition, which is not the case.
CR3022, an RBD-binding antibody, neutralizes SARS-CoV-2, possibly through its inhibition of VIM binding, while not preventing ACE2 binding. Further research is needed to identify other receptors that may interact with VIM, as earlier animal models with ACE2 knockdown showed a slight reduction in infection by SARS-CoV-2, whereas VIM knockdown led to a significant increase.
The widespread expression of VIM on mesenchymal derivatives, as well as on type 2 alveolar pneumocytes and nasal goblet cells, is increased during inflammation and viral infection. This may indicate “a potential role for VIM in infection of nasal and lung epithelial cells,” especially since ACE2 expression on lung alveolar cells and endothelial cells is quite low, requiring VIM to act as an attachment factor and enhance the viral entry.
“Taken together, the observations we present may lead to the development of new antiviral therapeutics combining therapies that inhibit interactions of both ACE2 and VIM with SARS-CoV-2.”
- Amraei, R., Xia, C., Oleknik, J., et al. (2022). Extracellular Vimentin Is an Attachment Factor That Facilitates SARS-Cov-2 Entry into Human Endothelial Cells. Proceedings of the National Academy of Sciences of the United States of America. doi:10.1073/pnas.2113874119.
Posted in: Medical Science News | Medical Research News | Disease/Infection News
Tags: ACE2, Angiotensin, Angiotensin-Converting Enzyme 2, Antibodies, Antibody, Blood, Blood Vessel, Brain, Cell, Cell Membrane, Coronavirus, Coronavirus Disease COVID-19, covid-19, Cytoplasm, Encephalitis, Endothelial cell, Enzyme, Heart, Inflammation, Japanese Encephalitis, Liver, Lungs, Membrane, Pandemic, Protein, Receptor, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Therapeutics, Thrombosis, Virus

Written by
Dr. Liji Thomas
Dr. Liji Thomas is an OB-GYN, who graduated from the Government Medical College, University of Calicut, Kerala, in 2001. Liji practiced as a full-time consultant in obstetrics/gynecology in a private hospital for a few years following her graduation. She has counseled hundreds of patients facing issues from pregnancy-related problems and infertility, and has been in charge of over 2,000 deliveries, striving always to achieve a normal delivery rather than operative.
Source: Read Full Article