Before undergoing surgeries and other invasive medical procedures, patients typically undergo anesthesia. Anesthesia consists in giving patients a class of drugs (i.e., anesthetics) that cause them to lose feeling in specific areas of the body (i.e., local anesthesia) or fully lose awareness during a procedure (i.e., general anesthesia). These anesthetics can be administered to patients via injection, inhalation, skin-numbing lotions, and other means.
In the past, doctors and medical researchers viewed general anesthesia as a passive process that could not be influenced or interrupted once anesthetic drugs were administered. More recently, however, studies showed that it is in fact an active brain process that can be experimentally controlled and acted on.
A research team at the Southern University of Science and Technology in China recently carried out a study investigating the processes underpinning brain states while under general anesthesia and those associated with the subsequent re-emergence of awareness. Their findings, published in Nature Neuroscience, highlight possible strategies that could help anesthesiologists to extend and deepen or shorten periods of anesthesia.
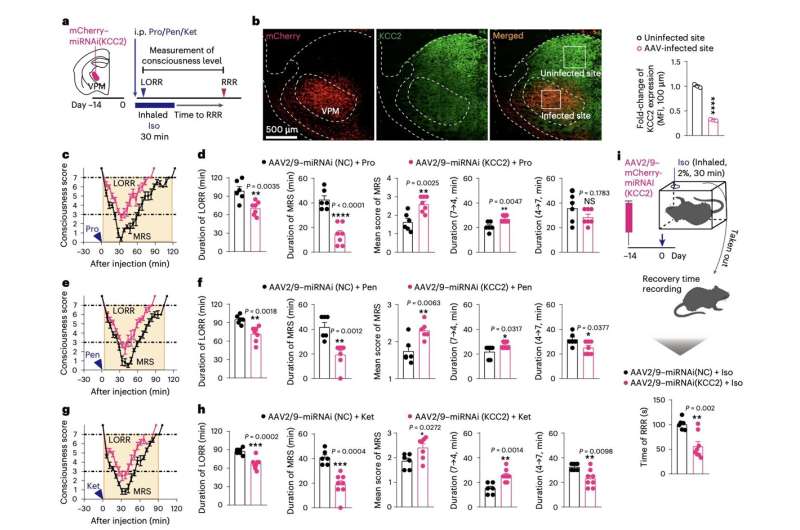
“We show in mice that, when the brain is forced into a minimum responsive state by diverse anesthetics, a rapid downregulation of K+/Cl− cotransporter 2 (KCC2) in the ventral posteromedial nucleus (VPM) serves as a common mechanism by which the brain regains consciousness,” Jiang-Jian Hu, Yuexin Liu and their colleagues wrote in their paper.
To examine the neural processes linked to the re-emergence of consciousness after anesthesia, the researchers carried out a series of experiments on adult mice. They gave the mice one of three different anesthetic drugs (i.e., ketamine, propofol and pentobarbital) and then looked at the molecular mechanisms that emerged while they were re-gaining awareness.
To assess the mice’s levels of consciousness, Hu, Liu and his colleagues measured the so-called loss of righting reflex (LORR), a point in which animals no longer act on their instinct to avoid laying with their stomach facing up. In addition, they observed the animals’ behavioral responses to external stimuli.
The team’s experiments yielded interesting results, identifying a new neuronal and molecular mechanism through which the brain re-gains consciousness after general anesthesia. They also showed that the ventral posteromedial nucleus (VPN), part of the thalamus, is a key brain region associated with re-emergence of consciousness.
“Ubiquitin-proteasomal degradation is responsible for KCC2 downregulation, which is driven by ubiquitin ligase Fbxl4,” Hu, Liu and their colleagues explained in their paper. “Phosphorylation of KCC2 at Thr1007 promotes interaction between KCC2 and Fbxl4. KCC2 downregulation leads to γ-aminobutyric acid type A receptor-mediated disinhibition, enabling accelerated recovery of VPM neuron excitability and emergence of consciousness from anesthetic inhibition. This pathway to recovery is an active process and occurs independent of anesthetic choice.”
Overall, the recent work by this team of researchers shows that the degradation of KCC2 transporter neurons located in the VPM, through the means of ubiquitin, a compound in living cells that contributes to the degradation of superfluous or faulty proteins in the brain, is a key step in the mice’s emergence of consciousness after general anesthesia. This key finding could potentially inform the development of strategies to wake patients who are in a vegetative state or minimally conscious state, which is a long-standing medical challenge.
More information:
Jiang-Jian Hu et al, Emergence of consciousness from anesthesia through ubiquitin degradation of KCC2 in the ventral posteromedial nucleus of the thalamus, Nature Neuroscience (2023). DOI: 10.1038/s41593-023-01290-y
Journal information:
Nature Neuroscience
Source: Read Full Article


