Everyone loves a sweet treat, even our cells. While we typically think of sugar as a component of our diet that gives us energy, sugars are also very important in guiding the activity of our cells. Sugar molecules can be modified and added to proteins on the outside of cells, where they help cells to perform certain tasks. The process of making these complex sugar molecules that coat cells is known as glycosylation.
“Glycosylation is like the clothing a cell wears. A person has clothes that go around them that do various things. That’s what glycosylation is for a cell,” explained Anand Mehta, D.Phil., senior associate dean of Research, SmartState Endowed Chair of Proteomic Biomarkers and professor in the Department of Cell and Molecular Pharmacology at MUSC.
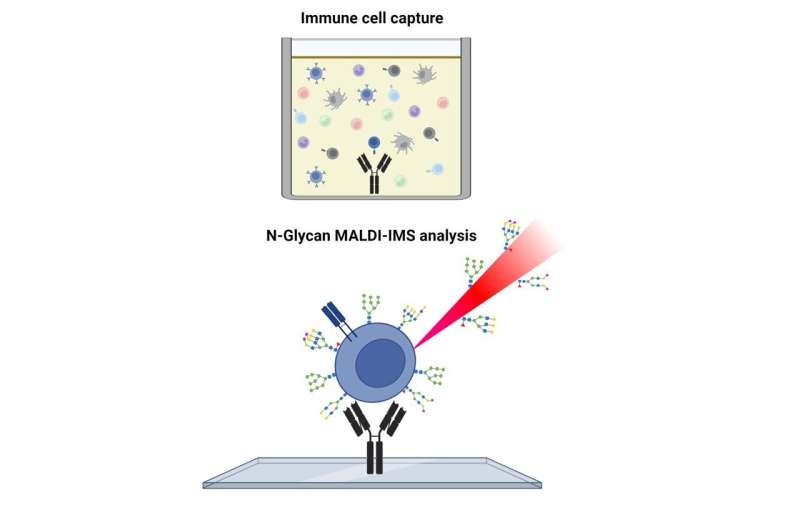
Mehta’s lab has developed a new method to investigate the sugars, called glycans, that coat immune cells. Their results, published in Analytical Chemistry, showed that different types of immune cells can be isolated from a mixture of cells and analyzed to determine the glycosylation of each cell type.
“These sugars play a major role in everything that happens in a cell,” said Mehta. “For the most part, we’ve had really poor methods to analyze those sugars. We developed a method to start to allow us to measure those sugar changes.”
The method that Mehta and his team developed is focused on capturing T-cells, a type of immune cell that helps to protect the body from infection and diseases, including cancer. Some T-cells help to activate other immune cells and trigger inflammatory responses, while others can directly kill infected or cancerous cells.
Previously, no methods were available to separate and analyze the glycosylation of these distinct cell populations efficiently. With their method, Mehta and his team are able to isolate these subtypes of cells quickly and determine how their sugar patterns differ in healthy versus diseased patients. These differences may be important to our understanding of how the body fights off cancer.
“Glycans can be used as biomarkers to figure out how the immune system is responding to a specific disease,” explained James Dressman, a Ph.D. candidate in Mehta’s laboratory who worked on this study.
Alterations in glycosylation are a hallmark of cancer that is not well-characterized. Patients with blood cancer will likely display alterations in their T-cell glycosylation patterns. Additionally, immune cell glycans can indicate whether these cells are active or resting.
Since the sugar coat on immune cells can alter their ability to kill diseased cells, increasing our understanding of immune cell glycosylation is extremely important. Immunotherapy cancer treatments involve modification of immune cells to make them more efficient cancer cell killers. Therefore, if scientists understand which sugars make T-cells best at killing specific types of cancer cells, more effective immunotherapies can be designed.
To perform T-cell glycan analysis, researchers add a mixture of immune cells to special microscope slides containing different regions that stick to specific types of T-cells. The sugars on the outside of these T-cells are then measured using a mass spectrometer, a complex machine that determines the mass and composition of small molecules.
An advantage of this method is its ability to isolate distinct cell populations from complex samples containing a mixture of cell types.
“It’s really exciting,” said Dressman. “It opens up new avenues for different questions that we can dig into.”
“We can look where no one had been able to look before,” added Mehta.
The first avenue Dressman and Mehta plan to investigate is how immune cell glycosylation changes in individuals with blood cancer. To do this, they will apply the new technique to immune cells in blood samples from patients with blood cancer and healthy individuals. The team hopes to identify blood biomarkers that will aid in early cancer detection. Additionally, they plan to optimize their technique to capture and study other types of immune cells.
Mehta and his team hope that one day this technique will be used in a clinical setting for diagnostic and prognostic tests. They anticipate that their technique will be useful in the development of more effective immunotherapy cancer treatments, predicting treatment outcomes and characterizing the immune response to different medications or vaccinations. Mehta hopes that others will see their publication and apply this technique to their studies.
“Science is all about learning from each other and making incremental improvements for the good of all,” he said.
More information:
James W. Dressman et al, Development of an Antibody-Based Platform for the Analysis of Immune Cell-Specific N-linked Glycosylation, Analytical Chemistry (2023). DOI: 10.1021/acs.analchem.3c00838
Journal information:
Analytical Chemistry
Source: Read Full Article


