Scientists believe that vaccinating the entire global population is the key to contain the ongoing coronavirus disease 2019 (COVID-19) pandemic. This pandemic is caused by the rapid outbreak of a ribonucleic acid (RNA) virus known as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
 Study: Synthetic Multiantigen MVA Vaccine COH04S1 Protects Against SARS-CoV-2 in Syrian Hamsters and Non-Human Primates. Image Credit: peterschrieber.media / Shutterstock.com
Study: Synthetic Multiantigen MVA Vaccine COH04S1 Protects Against SARS-CoV-2 in Syrian Hamsters and Non-Human Primates. Image Credit: peterschrieber.media / Shutterstock.com
Immune protection against SARS-CoV-2
To date, several COVID-19 vaccines have received emergency use authorization (EUA) from regulatory agencies in many countries across the world. Some of the vaccines that received EUA are based on adenovirus vectors, messenger ribonucleic acid (mRNA), and nanoparticle technologies. These vaccines target different antigenic forms of the SARS-CoV-2 spike (S) protein to elicit an immune response against SARS-CoV-2.
Although COVID-19 vaccines provided hope to contain the pandemic, the emergence of SARS-CoV-2 variants has prolonged the timeline. This is because some SARS-CoV-2 variants can evade the immune responses induced by vaccines or from previous natural infections.
The waning of neutralizing antibodies (NAb) produced by the vaccines has also challenged the durability of vaccine protection. Therefore, there is a need for an alternative vaccine based on various modifications of epitopes or antigen design to provide durable and long-lasting protection against SARS-CoV-2 variants of concern (VoC).
The primary immune correlate of protection includes blocking the S protein-angiotensin-converting enzyme 2 (ACE2) receptor interaction by NAb. However, several studies have indicated humoral and cellular immune responses are elicited via many antigens besides the S protein.
For instance, the nucleocapsid (N) protein is regarded as a dominant target to induce the production of antibodies and trigger T-cell responses. As the N protein is associated with a highly conserved region, it represents a favorable antigen target to generate durable and broadly reactive T-cells. Many studies using animal models have indicated the benefits of using N protein as a primary vaccine antigen.
The development of a multiantigenic SARS-CoV-2 vaccine using synthetic platforms
Previously, scientists have developed multiantigenic SARS-CoV-2 vaccine agents using synthetic platforms. Modified Vaccinia Ankara (MVA) vector, which is known as the backbone of vector vaccines, is marketed in the United States as Jynneos™ (Bavarian-Nordic).
MVA is a highly attenuated poxvirus vector that is commonly utilized for the production of vaccines for infectious diseases and cancer. MVA has many promising characteristics such as its safety profile in animals and humans, flexible delivery, and ability to elicit potent humoral and cellular immune responses to heterologous antigens.
A new study published on the bioRxiv* preprint server utilized MVA to develop vaccine candidates that were subsequently evaluated in animal models of congenital cytomegalovirus disease. This study indicated the efficacy of the vaccine among patients with solid tumors or those who had undergone stem cell transplantations.
In this study, researchers used a synthetic MVA (sMVA) platform to develop sMVA vectors co-expressing full-length S and N antigen sequences. These sequences exhibited promising immunogenicity in mice, as evidenced by their ability to trigger SARS-CoV-2 antigen-specific humoral and cellular immune responses with a high concentration of NAb.
One of the sMVA construct forms is COH04S1 and is known to be a clinical vaccine candidate. In a randomized, double-blinded, single-center, placebo-controlled Phase I clinical trial of healthy adults, researchers found that COH04S1 is safe and immunogenic. The current study assessed COH04S1 in a randomized, double-blinded, single-center Phase II trial in hematology patients who had received previously cellular therapy.
In their study, the researchers revealed that COH04S1 stimulated an immune response against SARS-CoV-2 in Syrian hamsters via intramuscular (IM) and intranasal (IN) vaccination. Also, non-human primates (NHP) were treated with single-dose (1D) and two-dose (2D) vaccination regimens. The results obtained in this study were in line with the clinical evaluation of this sMVA-based SARS-CoV-2 vaccine.
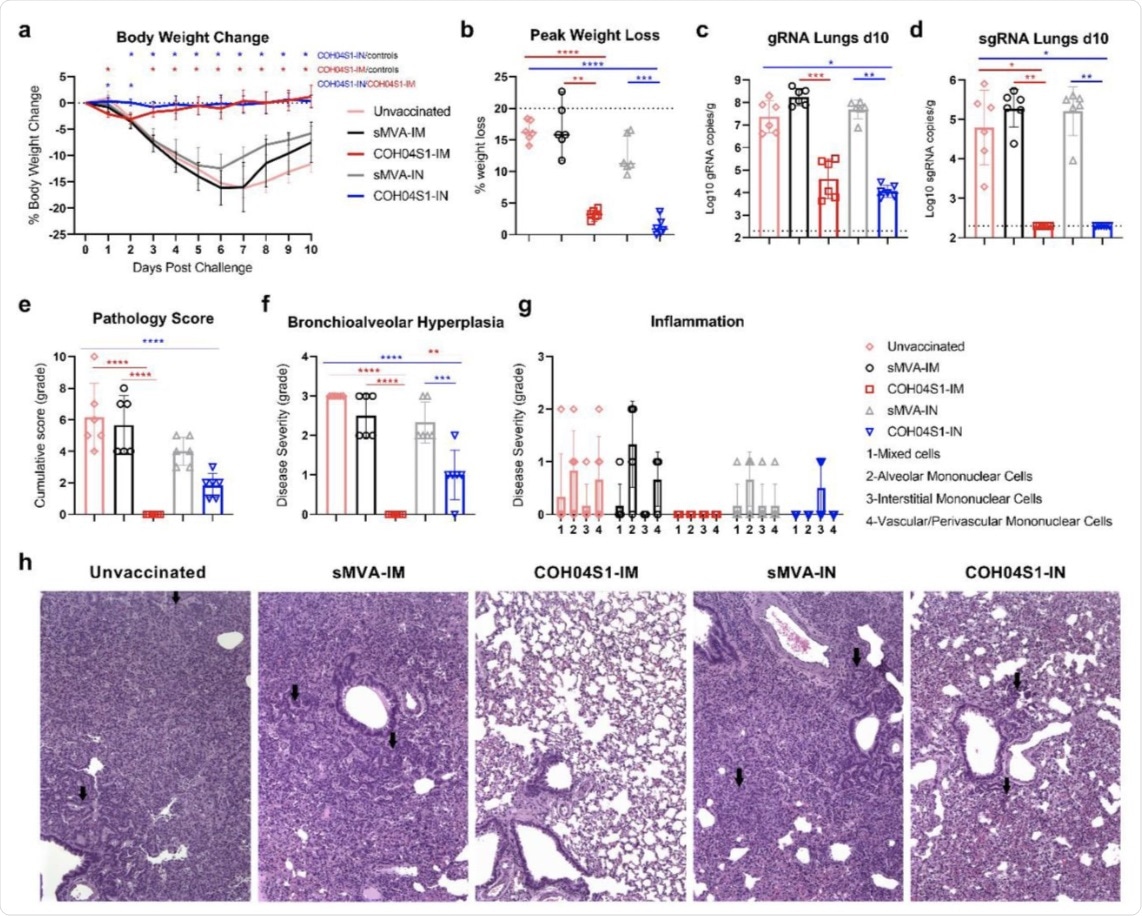 COH04S1-mediated vaccine protection in hamsters following sub-lethal SARS-CoV-2 challenge. a. Body weight change. Bodyweight of COH04S1-IM and COH04S1-IN-vaccinated animals as well as unvaccinated and sMVA-IM and sMVA-IN vector control animals was measured daily for 10 days post-challenge. Weight loss is reported as mean ±SD. Two-way ANOVA followed by Tukey’s multiple comparison test was used to compare group mean values at each timepoint. b. Maximal weight loss. Percentage of maximal weight loss is shown in single animals of vaccine and control groups. Lines and bars represent median values and 95% CI, respectively. The dotted line represents the maximum weight loss allowed before euthanasia. Peaks of weight loss in each group were compared using one-way ANOVA followed by Tukey’s multiple comparison test. c-d. Lung viral loads. SARS-CoV-2 genomic RNA (gRNA) and sub-genomic RNA (sgRNA) copies were quantified in lung tissue of vaccine and control groups at day 10 post-challenge by qPCR. Bars show RNA copies geometric mean ± geometric SD. Dotted lines represent a lower limit of detection. Kruskal-Wallis test followed by Dunn’s multiple comparison test was used. e-h. Histopathological findings. Hematoxylin/eosin-stained lung sections of COH04S1-vaccinated hamsters and control animals at day 10 post-challenge were evaluated by a board-certified pathologist and microscopic findings were graded based on the severity on a scale from 1 to 5 (Table S2). Panel e shows the cumulative pathology score of all histopathologic findings in each group. Panel f shows grading of bronchioalveolar hyperplasia disease severity in each group. One-way ANOVA followed by Holm-Sidak’s multiple comparison test was used. Panel g shows the severity of lung inflammatory microscopic findings based on 1-to-5 scaling of four inflammation types as indicated. Bars in e-g represent mean values ±SD. One-way ANOVA followed by Tukey’s multiple comparison test was used. In b-f *=0.05 < p <0.01, **=0.01 < p < 0.001, ***=0.001 < p < 0.0001, ****=p < 0.0001. Panel h shows representative images of histopathological findings in lung sections of COH04S1-vaccinated animals and control animals. Black arrows indicate moderate and mild bronchioalveolar hyperplasia in lung sections of sMVA-IM and sMVA-IN control animals as well as COH04S1-IN animals. Black arrows in lung sections of unvaccinated control animals indicate hyperplastic alveolar cells. 10x magnification.
COH04S1-mediated vaccine protection in hamsters following sub-lethal SARS-CoV-2 challenge. a. Body weight change. Bodyweight of COH04S1-IM and COH04S1-IN-vaccinated animals as well as unvaccinated and sMVA-IM and sMVA-IN vector control animals was measured daily for 10 days post-challenge. Weight loss is reported as mean ±SD. Two-way ANOVA followed by Tukey’s multiple comparison test was used to compare group mean values at each timepoint. b. Maximal weight loss. Percentage of maximal weight loss is shown in single animals of vaccine and control groups. Lines and bars represent median values and 95% CI, respectively. The dotted line represents the maximum weight loss allowed before euthanasia. Peaks of weight loss in each group were compared using one-way ANOVA followed by Tukey’s multiple comparison test. c-d. Lung viral loads. SARS-CoV-2 genomic RNA (gRNA) and sub-genomic RNA (sgRNA) copies were quantified in lung tissue of vaccine and control groups at day 10 post-challenge by qPCR. Bars show RNA copies geometric mean ± geometric SD. Dotted lines represent a lower limit of detection. Kruskal-Wallis test followed by Dunn’s multiple comparison test was used. e-h. Histopathological findings. Hematoxylin/eosin-stained lung sections of COH04S1-vaccinated hamsters and control animals at day 10 post-challenge were evaluated by a board-certified pathologist and microscopic findings were graded based on the severity on a scale from 1 to 5 (Table S2). Panel e shows the cumulative pathology score of all histopathologic findings in each group. Panel f shows grading of bronchioalveolar hyperplasia disease severity in each group. One-way ANOVA followed by Holm-Sidak’s multiple comparison test was used. Panel g shows the severity of lung inflammatory microscopic findings based on 1-to-5 scaling of four inflammation types as indicated. Bars in e-g represent mean values ±SD. One-way ANOVA followed by Tukey’s multiple comparison test was used. In b-f *=0.05 < p <0.01, **=0.01 < p < 0.001, ***=0.001 < p < 0.0001, ****=p < 0.0001. Panel h shows representative images of histopathological findings in lung sections of COH04S1-vaccinated animals and control animals. Black arrows indicate moderate and mild bronchioalveolar hyperplasia in lung sections of sMVA-IM and sMVA-IN control animals as well as COH04S1-IN animals. Black arrows in lung sections of unvaccinated control animals indicate hyperplastic alveolar cells. 10x magnification.
The IM and IN vaccination of Syrian hamsters with COH04S1 elicited strong Th1-biased antigen-specific humoral immunity, as well as cross-NAbs. The animals were found to be protected from reduced weight, lower respiratory tract infection, and lung injury post IN SARS-CoV-2 challenge.
The researchers also found that 1D and 2D vaccines induced strong antigen-specific binding antibodies, NAbs, and Th1-biased T-cells. These immune cells strongly provided protection to both upper and lower respiratory tract infections.
Conclusion
The current study developed COH04S1, which is a synthetic multiantigen MVA-based SARS-CoV-2 vaccine that targets the SARS-CoV-2 S and N antigens. The efficacy of the newly developed vaccine was determined using animal models.
The current research revealed that the COH04S1 vaccine provides protection via different routes and dose regimens. The results complemented the ongoing clinical trials of the multiantigen SARS-CoV-2 vaccine.
COH04S1 represents a second-generation COVID-19 vaccine candidate that could be used alone or in combination with other existing vaccines to elicit protective immune responses against SARS-CoV-2. The authors of this study are optimistic that this vaccine would establish a long-term and stable immune response to prevent COVID-19.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Chiuppesi, F., Nguyen, V. H., Park, Y., et al. (2021). Synthetic Multiantigen MVA Vaccine COH04S1 Protects Against SARS-CoV-2 in Syrian Hamsters and Non-Human Primates. bioRxiv. doi:10.1101/2021.09.15.460487. https://www.biorxiv.org/content/10.1101/2021.09.15.460487v1.
Posted in: Drug Trial News | Medical Research News | Disease/Infection News
Tags: ACE2, Adenovirus, Angiotensin, Angiotensin-Converting Enzyme 2, Antibodies, Antigen, Cancer, Cell, Clinical Trial, Coronavirus, Coronavirus Disease COVID-19, Cytomegalovirus, Efficacy, Enzyme, Euthanasia, Genomic, Hematology, Hyperplasia, Immune Response, immunity, Infectious Diseases, Inflammation, Magnification, Nanoparticle, Pandemic, Pathology, Placebo, Protein, Receptor, Research, Respiratory, Respiratory Tract Infections, Ribonucleic Acid, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, T-Cell, Vaccine, Virus, Weight Loss

Written by
Dr. Priyom Bose
Priyom holds a Ph.D. in Plant Biology and Biotechnology from the University of Madras, India. She is an active researcher and an experienced science writer. Priyom has also co-authored several original research articles that have been published in reputed peer-reviewed journals. She is also an avid reader and an amateur photographer.
Source: Read Full Article