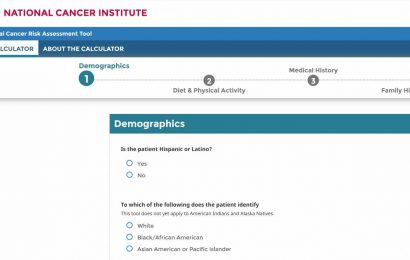
A commercially available genomic test may help oncologists better determine which patients with recurrent prostate cancer may benefit from hormone therapy, according to new research from the Johns Hopkins Kimmel Cancer Center and 15 other medical centers.
Researchers studied prostate cancer samples from 352 participants in the NRG/RTOG 9601 clinical trial, which compared radiation therapy alone with radiation therapy combined with hormone therapy. The investigators found that the Decipher test, which measures the activity of 22 genes among seven known cancer pathways, independently estimated the participants’ risk of metastasis, death from prostate cancer and overall survival. Researchers say it also guided treatment recommendations for recurrence of prostate cancer after surgery, helping identify patients most likely to benefit from hormone therapy.
Results were published online Feb. 11 in the journal JAMA Oncology.
“The findings may be practice-changing, and will give oncologists additional information to help guide decisions on whether to offer patients hormone therapy,” says senior study author Phuoc Tran, M.D., Ph.D., professor of radiation oncology and molecular radiation sciences and co-director of the Cancer Invasion and Metastasis Program at the Johns Hopkins Kimmel Cancer Center.
For the study, Tran and colleagues studied prostate cancer samples from 352 men whose prostate cancer recurred after surgery and who participated in the NRG/RTOG 9601 clinical trial of radiation therapy between March 1998 and March 2003. The participants were randomized to receive radiation with hormone therapy (150 milligrams of bicalutamide daily for two years) or radiation therapy without hormone therapy. The researchers ran genetic information called ribonucleic acid (RNA) from the tumor tissue through the Decipher test, which evaluates the activity of 22 genes to predict how aggressive the cancer is and its chances of metastasis.
The test uses “genomic classifier scores” determined from the genomic characteristics of the tumor to stratify patients into three groups. Lower scores correlate with a more favorable prognosis. Of the participants, 148 patients (42%) had low genomic classifier scores, below .45; 132 (38%) had intermediate genomic classifier scores, between .45 and .60; and 72 (20%) had high genomic classifier scores, above .60. These genomic classifiers helped predict the risk of distant metastases, prostate cancer-specific death and overall survival, even after adjusting for participants’ age, race/ethnicity, Gleason score, T stage of T1 to T4 to classify the extent of tumor spread, margin status, prostate-specific antigen level and whether they were receiving hormone therapy.
Specifically, patients with intermediate and high genomic classifier scores had an 88% increased risk of distant metastases versus those with low genomic classifier scores. The test also demonstrated that patients with lower genomic classifier scores had a 2.4% improvement in overall survival 12 years after treatment with radiation and hormone therapy, compared with an 8.9% improvement among those with higher genomic classifier scores. Additionally, patients receiving radiation therapy soon after recurrence benefited more from hormone therapy. Those with higher genomic classifier scores derived an 11.2% improvement in 12-year occurrence of distant metastasis and a 4.6% improvement in overall survival from taking hormone therapy.
“Patients with lower scores do better overall, but they do not benefit as much from adding on hormone therapy because they are low risk. Patients with higher scores have worse disease and, therefore, benefit the most from adding the hormone therapy,” explains Tran.
Source: Read Full Article