A study conducted by researchers from Maryland in the United States has found no correlation between the symptoms that develop following vaccination against coronavirus disease 2019 (COVID-19) and the antibody responses that are generated against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The team’s analysis of 206 adults who received the Pfizer-BioNTech BNT162b2 vaccine found no correlation between vaccine-associated symptom severity scores and the magnitude of vaccine-induced antibody responses to SARS-CoV-2.
The researchers from the Uniformed Services University of the Health Sciences in Bethesda, the Naval Medical Research Center in Silver Spring, and the Frederick National Laboratory for Cancer Research say that some media outlets and medical professionals have frequently referred to the presence of post-immunization symptoms as a sign that a COVID-19 vaccine is “working.”
This incorrectly implies that a lack of symptoms means that a vaccine has not induced the appropriate antibody responses.
However, Edward Mitre and colleagues say the current study findings suggest that individuals who do not develop symptoms can be reassured that this does not mean the vaccine “did not work.”
The team also reports that symptoms were inversely correlated with age and weight, were more common among women, and occurred more frequently following a second vaccine dose.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes pee review.
Relationship between post-vaccination symptoms and antibody responses is unclear
The mass rollout of vaccines that protect against SARS-CoV-2 infection currently represents the most promising approach to bringing the COVID-19 pandemic under control.
One feature shared by both the Pfizer-BioNTech BNT162b2 and Moderna mRNA-1273 vaccines is the high level of reactogenicity. The majority of participants from phase 1 to 3 studies reported both local and systemic reactions.
Coggins and his team say it has become commonplace for media outlets and medical professionals to state that the development of symptoms indicates that a vaccine is “working.”
“Although this statement is fundamentally true because vaccines ‘work’ by inducing inflammatory responses, it also implies incorrectly that a lack of symptoms post-vaccination may indicate an absence of appropriate antiviral antibody responses,” say the researchers.
“Notably, there is little data demonstrating correlations between vaccine-induced symptoms and antibody titers with any vaccine platforms,” they add.
What did the current study involve?
The team assessed whether adverse effects induced by first and second doses of the Pfizer-BioNTech BNT162b2 vaccine were associated with the magnitude of the anti-SARS-CoV-2 antibody response among participants enrolled in the Prospective Assessment of SARS-CoV-2 Study (PASS).
PASS is an observational, longitudinal cohort study of healthcare workers that was initiated in August of 2020 to assess clinical and immunologic responses to SARS-CoV-2 infection and vaccination.
Participants completed a vaccine-associated symptom questionnaire one month after receiving each vaccination. In addition, Immunoglobulin G (IgG) antibodies against the SARS-CoV-2 spike protein and spike receptor-binding domain (RBD) were measured using microsphere-based multiplex immunoassays.
The spike protein mediates the initial stage of the infection process when its RBD binds to the host cell receptor angiotensin-converting enzyme 2 (ACE2). This spike RBD is the primary target of antibodies following SARS-CoV-2 infection or vaccination.
What did the study find?
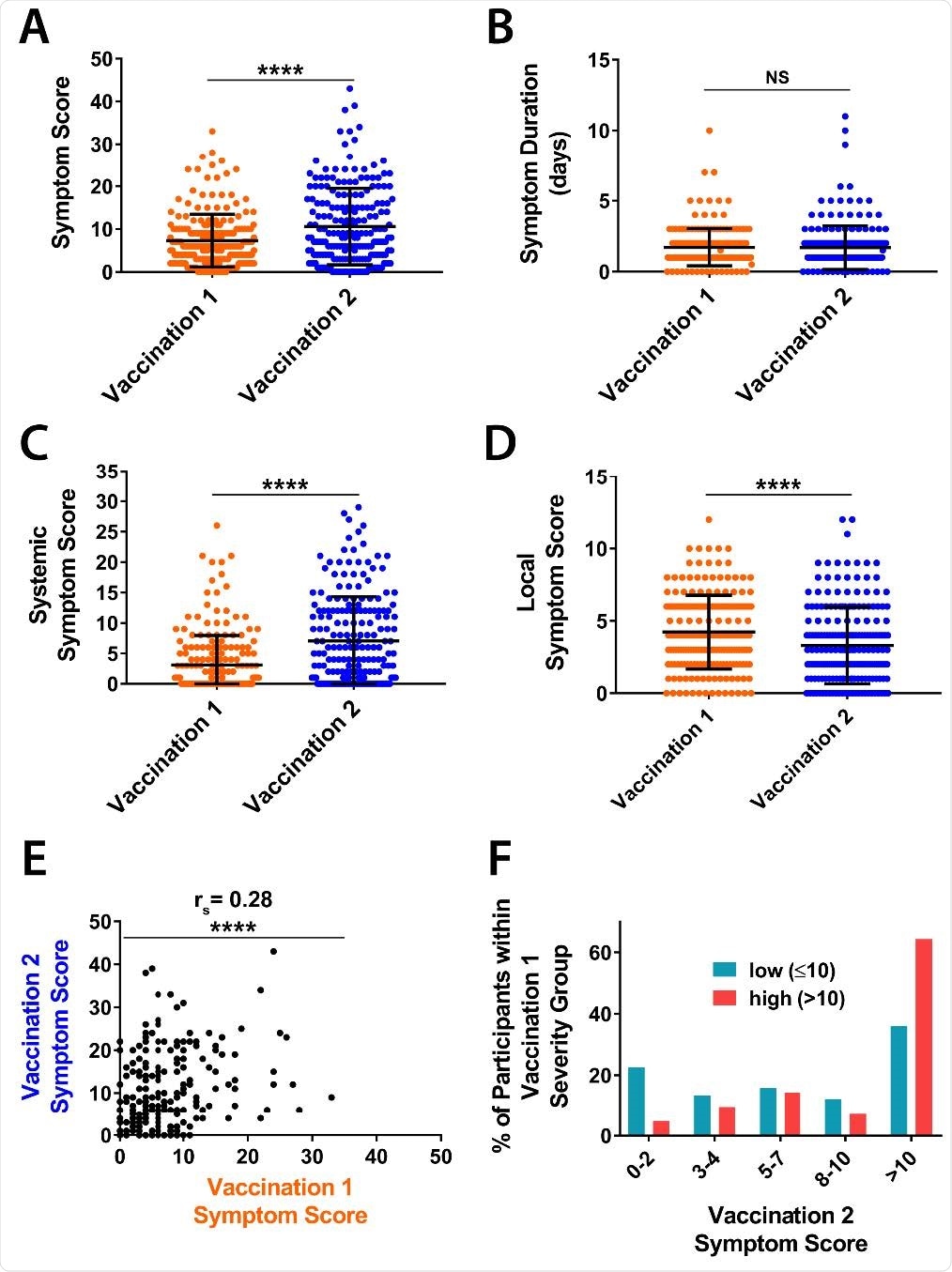
Among 206 of 271 PASS participants (aged a median of 41.5 years) who were seronegative for SARS-CoV-2 prior to vaccination, no correlations between vaccine-associated symptom severity scores and vaccine-induced IgG titers were identified one month after the first or second vaccine dose.
Individuals with either high or low symptom scores had similar levels of both spike-specific and RBD-specific IgG antibodies.
Coggins and colleagues say this lack of correlation was maintained even following adjustment for age, weight and sex.
“Lack of post-vaccination symptoms following receipt of the BNT162b2 vaccine does not equate to a lack of vaccine-induced antibodies one month after vaccination,” writes the team.
High symptom scores were more common among women
The researchers also found that high post-vaccination symptoms scores were more common among women and were inversely correlated with age and weight.
“Younger age, female sex, and lower weight were all associated with higher symptom scores,” they write.
Coggins and the team point out that women evaluated in a large-scale UK-based study were also found to have more symptoms than men following BNT162b2 vaccination.
“The determination of whether women and/or low weight individuals have greater BNT162b2- associated adverse events may be informed by additional cohort studies,” they suggest.
The study also found that the mean symptom score reported following the second vaccination was significantly higher than for the first vaccination, at 10.6 versus 7.3.
Furthermore, there was a positive correlation between symptom scores following the first and second vaccination.
Individuals who had a high symptom score following the first dose were almost twice as likely to have substantial symptoms following the second dose than those who had a low symptom score following the first dose.
What did the authors conclude?
The researchers say the study shows that the BNT162b2 vaccine is commonly associated with the development of symptoms.
However, “symptoms are greater after the second vaccination, are more common in younger individuals, and do not correlate with vaccine-induced antiviral IgG titers,” they write.
“These findings suggest that patients receiving the BNT162b2 vaccine should be reassured that a lack of symptoms does not necessarily equate to a lack of desired vaccine function,” concludes the team.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Coggins S, et al. Adverse effects and antibody titers in response to the BNT162b2 mRNA COVID-19 vaccine in a prospective study of healthcare workers. medRxiv, 2021. doi: https://doi.org/10.1101/2021.06.25.21259544, https://www.medrxiv.org/content/10.1101/2021.06.25.21259544v1
Posted in: Medical Research News | Disease/Infection News
Tags: ACE2, Angiotensin, Angiotensin-Converting Enzyme 2, Antibodies, Antibody, Cancer, Cell, Coronavirus, Coronavirus Disease COVID-19, Enzyme, Healthcare, Immunization, Immunoassays, Immunoglobulin, Laboratory, Medical Research, Pandemic, Protein, Receptor, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Vaccine

Written by
Sally Robertson
Sally first developed an interest in medical communications when she took on the role of Journal Development Editor for BioMed Central (BMC), after having graduated with a degree in biomedical science from Greenwich University.
Source: Read Full Article


