Immunotherapies, such as checkpoint inhibitor drugs, have made worlds of difference for the treatment of cancer. Most clinicians and scientists understand these drugs to act on what’s known as the adaptive immune system, the T cells and B cells that respond to specific threats to the body.
New research from an international team co-led by George Hajishengallis of the University of Pennsylvania School of Dental Medicine suggests that the innate immune system, which responds more generally to bodily invaders, may be an important yet overlooked component of immunotherapy’s success.
Their work, published in the journal Cell, found that “training” the innate immune system with β-glucan, a compound derived from fungus, inspired the production of innate immune cells, specifically neutrophils, that were primed to prevent or attack tumors in an animal model.
“The focus in immunotherapy is placed on adaptive immunity, like checkpoint inhibitors inhibit the interaction between cancer cells and T cells,” says Hajishengallis, a co-senior author on the work. “The innate immune cells, or myeloid cells, have not been considered so important. Yet our work suggests the myeloid cells can play a critical role in regulating tumor behavior.”
The current study builds on earlier work published in Cell by Hajishengallis and a multi-institutional team of collaborators, which showed that trained immunity, elicited through exposure to exposure to the fungus-derived compound β-glucan, could improve immune recovery after chemotherapy in a mouse model.
In that previous study, the researchers also showed that the ‘memory’ of the innate immune system was held within the bone marrow, in hematopoetic stem cells that serve as precursors of myeloid cells, such as neutrophils, monocytes, and macrophages.
The team next wanted to get at the details of the mechanism by which this memory was encoded. “The fact that β-glucan helps you fight tumors doesn’t necessarily mean it was through trained immunity,” says Hajishengallis.
To confirm that link, the researchers isolated neutrophils from mice that had received the innate immune training via exposure to β-glucan and transferred them, along with cells that grow into melanoma tumors, to mice that had not received β-glucan. Tumor growth was significantly dampened in animals that received cells from mice that had been trained.
To further support this link between myeloid precurors and the protective quality of trained immunity, the scientists performed bone marrow transplants, transferring bone marrow cells from “trained” mice to untrained mice that had been irradiated, effectively eliminating their own bone marrow.
When challenged later, the mice that were recipients of bone marrow from trained mice fought tumors much better than those that received bone marrow from untrained mice. “This is innate immune memory at work,” said Technical University Dresden’s Triantafyllos Chavakis, a long-term collaborator of Hajishengallis and co-senior author of the study.
The experiment relied on the memory of bone marrow precursors of neutrophils of the trained donor mice, which were transferred by transplantation to the recipient mice and gave rise to neutrophils with tumor-killing ability.
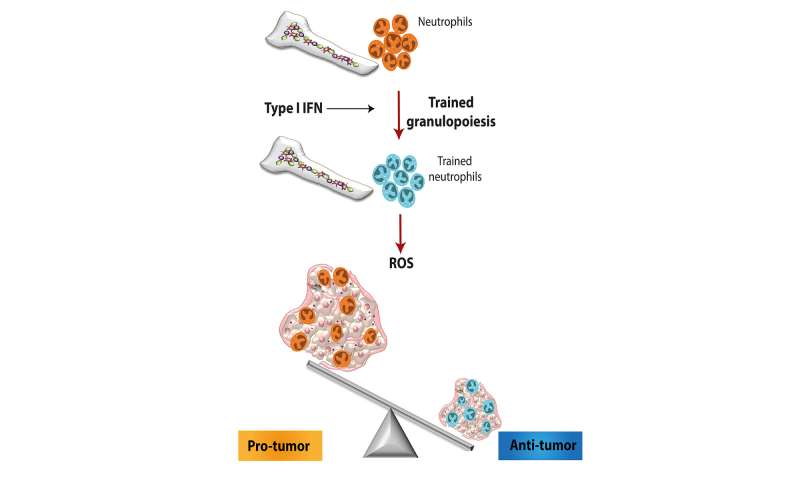
The researchers found that the antitumor activity likely resulted from trained neutrophils producing higher levels of reactive oxygen species, or ROS, than did untrained neutrophils. ROS can cause harm in certain contexts but in cancer can be beneficial, as it acts to kill tumor cells.
Looking closely at the myeloid precursors in the bone marrow of trained animals, the team found significant changes in gene expression that biased the cells toward making neutrophils, specifically a type associated with anti-tumor activity, a classification known as tumor-associated neutrophils type I (TAN1).
Further investigation revealed that these changes elicited by innate immune training cause an epigenetic rewiring of bone marrow precursor cells, changes that acted to make certain genes more accessible to being transcribed and also pointed to the Type I interferon signaling pathway as a likely regulator of innate immune training. Indeed, mice lacking a receptor for Type I interferon couldn’t generate trained neutrophils.
β-glucan is already in clinical trials for cancer immunotherapy, but the researchers say this finding suggests a novel mechanism of action with new treatment approaches.
Source: Read Full Article