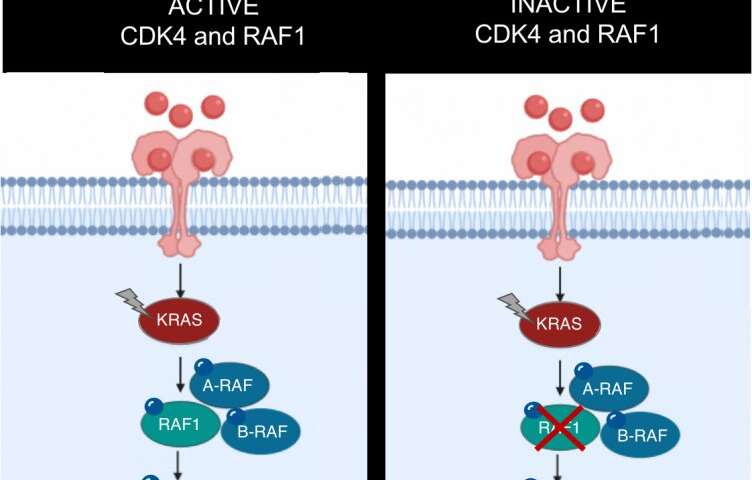
The KRAS oncogene is involved in at least one fifth of all human cancers: KRAS mutations are directly responsible for 32% of lung tumors and 96% of pancreatic tumors. However, after more than thirty years of research to date, there are still no effective therapeutic strategies against this oncogene. For this reason, much of the research conducted seeks to identify other molecules that display therapeutic activity along the KRAS signaling pathway. Rising to this challenge, researchers from the Experimental Oncology Group of the Spanish National Cancer Research Centre (CNIO) have achieved complete remission in 25% of lung tumors induced by this oncogene in mice following genetic inactivation of CDK4 and RAF1, which opens new venues for the development of future treatments. This finding has been published by PNAS, the journal of the American Academy of Sciences.
In this project, the CNIO team explored an approach that consists of inactivating two genes that are part of the KRAS signaling pathway: CDK4 and RAF1. “And this strategy has worked,” says Monica Musteanu, one of the leading authors of the study. By working with mouse models for lung cancer caused by a combination of two mutations—activation of the KRAS oncogene and elimination of the tumor suppressor gene p53, two of the most frequently mutated genes in human cancer—they have managed to shrink 100% of the tumors, and a quarter of them have remitted completely. In addition, the researchers have confirmed in healthy mice that this therapeutic strategy does not compromise normal functioning of the body.
Even so, as is often found in clinical practice, a percentage of the tumors were able to survive in the absence of CDK4 and RAF1. However, the researchers were able to identify the molecular mechanisms that triggered this resistance and prevented total tumor remission: activation of the PI3K pathway, essential in cancer, and the silencing of several tumor suppressor genes by means of methylation. “Both mechanisms can be therapeutically neutralized: using PI3K inhibitors, on the one hand, and on the other, through the selective demethylation of tumor suppressor genes to reactivate their function,” explains Laura de Esteban, the lead author of the research published by PNAS.
To carry out this study, the CNIO team used a mouse model that accurately reproduces the human disease, inducing aggressive lung tumors by activating the KRAS oncogene and deactivating the p53 tumor suppressor, a genetic combination responsible for a high percentage of human lung adenocarcinomas. Then, once the tumor has formed, genes are inactivated in the mouse systemically in the same way that a patient would receive treatment after diagnosis.
The findings shed light on the development of new treatments for tumors with KRAS mutations and indicate the importance of developing inhibitors specifically against RAF1, because the inhibitors developed so far have not made it past phase I on account of their high toxicity.
Source: Read Full Article


