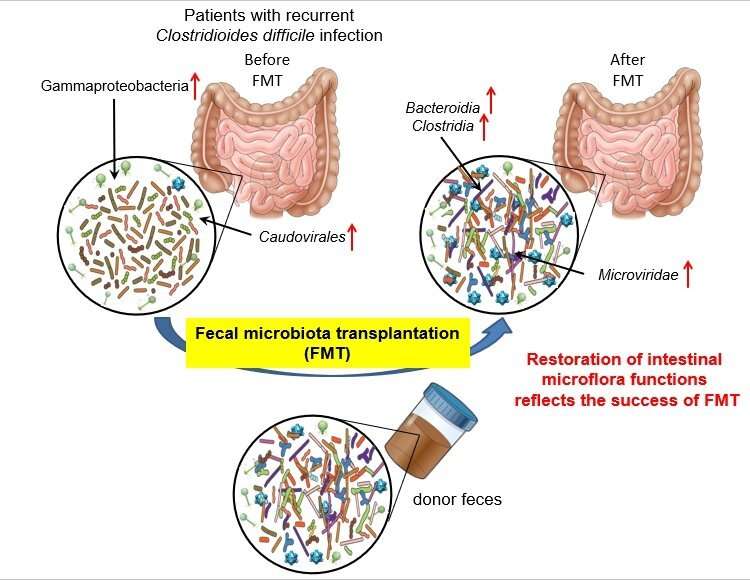
In a study published in Gastroenterology—Researchers at Osaka City University and the Institute for Medical Science, The University of Tokyo, in collaboration with Brigham and Women’s Hospital in Boston, report the intestinal bacterial and viral metagenome information from the fecal samples of patients with recurrent Clostridioides difficile infection (rCDI). This comprehensive analysis reveals the bacteria and phages involved in pathogenesis in rCDI, and their remarkable pathways important for the recovery of intestinal flora function.
Clostridioides difficile infection (rCDI) occurs in the gut and is caused by the Gram-positive, spore-forming anaerobic bacterium, C. difficile when its spores attach to fecal matter and are transferred from hand to mouth by health care workers. Patients undergoing antibiotic treatment are especially susceptible as the microorganisms that maintain a healthy gut are greatly damaged by the antibiotics.
Treatment of rCDI involves withdrawing the causative antibiotics and initiating antibiotic therapy, although this can be very challenging. Fecal microbiota transplantation (FMT) is considered an effective alternative therapy as it addresses the issue from the ground up by replacing the damaged microflora with a healthy one through a stool transplant.
However, two deaths caused by antibiotic-resistant bacterial infections after FMT were reported in 2019, suggesting that a modification of FMT or alternatives are required to resolve safety concerns surrounding the treatment.
Researchers at Osaka City University and the Institute for Medical Science, University of Tokyo tackled this challenge head on in a great study now published in Gastroenterology.
Using their original analysis pipeline reported in 2020, the researchers obtained intestinal bacterial and viral metagenome information from the fecal samples of nine rCDI patients from Brigham and Women’s Hospital in Boston who successfully had a FMT. They revealed the bacteria and phages involved in the pathogenesis of rCDI and the remarkable pathways important for the recovery of intestinal flora function.
By revealing how the bacteriome and virome in the intestine work together as an organ, the research team was able to show how FMT can be as safe as swapping out a bad organ with a good one.
“Intestinal microbiota should definitely be treated as an organ!” says principal investigator Professor Satoshi Uematsu. “FMT drastically changed the intestinal bacteriome and virome and is sure to restore the intestinal bacterial and viral functions.”
Source: Read Full Article


