Molecular mechanism of infection in cells: The cellular entry of coronaviruses depends on the binding of proteins with viral spicules (S) to the cellular receptors and the priming of the S protein by the host cell proteases. The SARS-CoV-2 virus has been shown to use the ACE2 receptor from the level of pulmonary alveoli, for entry and an enzyme, TMPRSS2 serine protein for initiating protein S. A TMPRSS2 inhibitor approved for clinical use blocked entry and could be a treatment option. Some results revealed an important communication between SARS-CoV-2 and SARS-CoV. About 80% of COVID-19 infections are mild.
Patients with SARS-CoV-2 in convalescents exhibit a neutralizing antibody response that can be detected even at 24 months after infection and is largely directed against protein S. In addition, experimental SARS vaccines, including recombinant S protein and inactivated virus induce responses to neutralizing antibodies. Although confirmation of the infectious virus is recent, our results indicate that neutralizing high antibody responses against SARS-S may provide some protection against SARS-CoV-2 infection.
There are two types of rapid tests. One is the serological, immunological test (a drop of blood is taken from the finger, through the sting) and offers the results in 20-30 minutes. The antibody test produces positive results, in the case of infection, SARS-CoV-2, only after a few days if the antibodies have formed in the blood. These tests are not suitable for the detection of active infections in the early phase of the disease.
This assay determines the presence of specific antibodies against the new subtype coronavirus (SARS-CoV-2), respectively IgM and IgG. It should be noted, however, that this type of rapid test does not provide an early diagnosis, as it is positive 3-5 days after the clinical onset of the disease. In other words, in the case of an asymptomatic person, who is more than 3-5 days after the onset of the disease, the test can be positive.
The second type of rapid test is the one obtained from the nasopharyngeal exudate, gives results in 20-30 minutes and aims to identify the viral antigen, being similar to the rapid flu tests. These rapid tests are more effective than serological tests because they are useful for both early diagnosis and early diagnosis of new coronavirus infection. No testing method is perfect.
Through the Immune Linked Enzymes Assay (Elisa), the IgM or IgG antibody binds both SARS-CoV and SARS-CoV-2 with high affinity.
Ancillary tests in the case of CoV-19 disease showed different values compared to the reference values in the case of Hematology with Leukocyte Formula, 5-Diff, (Lymphopenia Neutrophilia), Number of Leukocytes, Thrombocytes, Reactive Protein C, Fibrinogen, VSH, Interleukin IL- 6, Procalcitonin, Albumen, Urea, Creatinine, Acid Uric, AST (TGO), ALT (TGP), LDH, FAL, Total Bilirubin, Thromboplastin Time, Thrombin Time (TT) and D-Dimers with a predisposition.
Sequential Gene Method (NGS) Using SmartXGeneNGS Analyzer
SmartXGene intends to introduce automated sequencing analysis software into the future and impact of copy variants on a single 16S rRNA genome. In order to minimize the proportion of negative results, it is recommended to further test the samples collected from the respiratory apparatus in very suspicious cases and to check the sample quality.
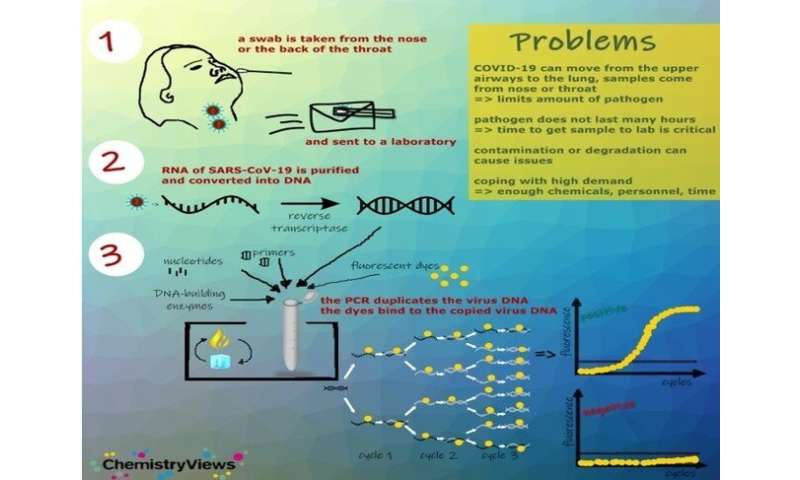
Reverse transcription-polymerase chain reaction assay (RT-PCR)
RT-PCR assays identify the genetic fingerprint of the virus (viral genome), that is, RNA. There are several PCR platforms, the sample can be taken from respiratory secretions, nasopharyngeal exudate or bronchial aspirate.
The waiting time varies, depending on the platform used, from 45 minutes to 6 hours.
Conclusions
Source: Read Full Article


