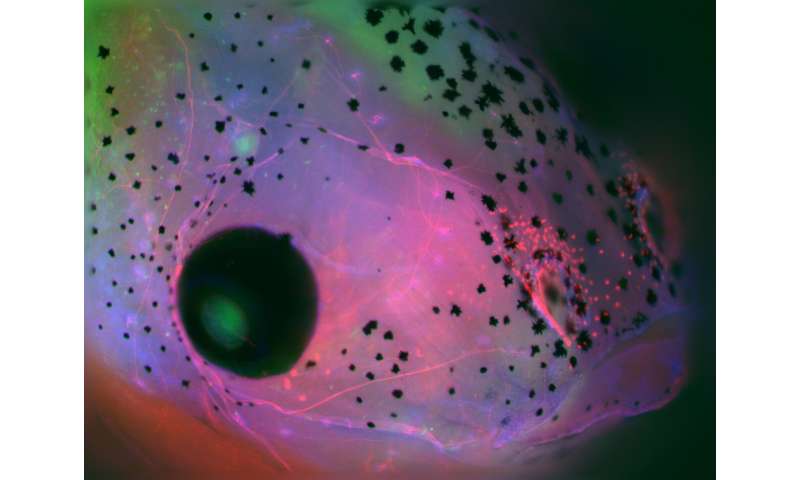
Researchers led by biologists at Tufts University have discovered that the brains of developing embryos provide signals to a nascent immune system that help it ward off infections and significantly improve the embryo’s ability to survive a bacterial challenge. Using frog embryos, which continue to develop with their brains removed, the researchers found that embryos without a brain are not able to marshall the forces of immune cells to an injury or infection site, leading the embryo to succumb to an infection more quickly. By contrast, the presence of a brain crucially helps direct immune cells to the site of injury to overcome the bacterial threat. The study was published today in NPJ Regenerative Medicine.
In a developing embryo, both brain and immune system are not fully formed. The immune system, for its part, consists mostly of an “innate” system of cells that respond immediately to infection and do not require training or produce antibodies. Nevertheless, these cells require signals that prompt them to move toward an infection site and trigger a response.
The research team found that the brain appears to contribute to the signals that guide the nascent immune system. When brainless frog embryos were infected with E. coli, only about 16% of embryos survived, while the presence of a brain protected more than 50% from the infection. By following markers of immune cells, researchers confirmed that the effect is not due to the missing brain somehow hampering immune system development because the composition of the immune cells remained the same with or without a brain. Instead, they found that the effect was due to the brain sending signals to the immune cells to move toward the site of an infection.
“We found that macrophages -innate immune system cells that can swallow up bacteria and destroy them to reduce the burden of an infection do not migrate appropriately without the presence of the brain” said Michael Levin, Vannevar Bush Professor of Biology at Tufts University’s School or Arts and Sciences and Associate Faculty at Harvard’s Wyss Institute, director of the Allen Discovery Center at Tufts and corresponding author of the study. “Without the brain and its neurotransmitter signals, gene expression and innate immune system activity go awry, resulting in increased susceptibility to bacterial pathogens. “
Other roles for the embryonic brain signaling during infection may include inducing cellular responses, for example preventing cell death or reducing inflammation, that help protect against the harmful effects of the infection.
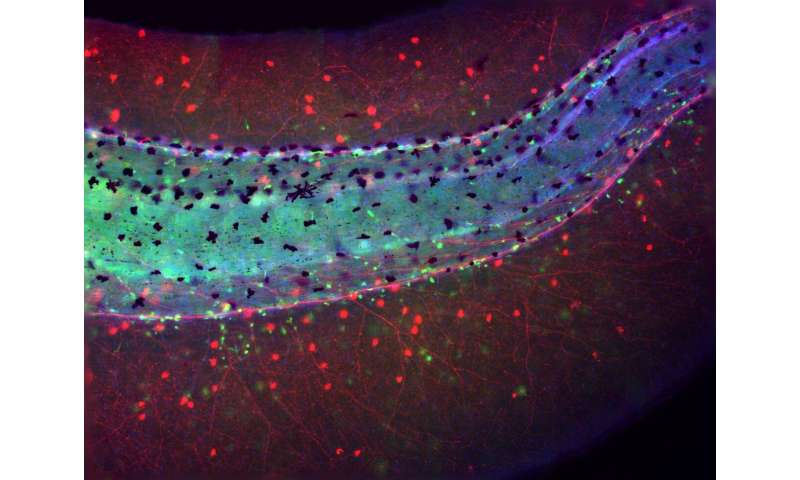
Immune system abberations were also observed in brainless embryos that were further developed. When the researchers tracked myeloid cells, a class of immune cells that includes macrophages, neutrophils and others, after an injury, they found that the myeloid cells in brainless embryos gathered in locations far from the injury site. By contrast, myeloid cells in normal embryos with intact brains would pile up at the injury site to assist in healing. In fact, in the brainless embryo, the myeloid cells tended to cluster around abnormal, disorganized peripheral nerve networks, also a by-product of brain absence, as demonstrated in earlier studies.
An examination of aberrations in genetic expression in brainless embryos also pointed to the reduction of the neurotransmitter dopamine (a signaling chemical used in the brain for learning and motivation), and that dopamine may play a role in activating immune cells to migrate in the early stages of an infection. The absence of an immune cell quorum at the infection site leads the brainless embryos to become more susectible to its lethal effects.
Source: Read Full Article


